03-13-03 00:00
No 416280
In this story I am writing I wish to be as real to life as possible-My question, if someone had GBL stored in a beaker and wished to stopper the beaker using a cork, would the gbl if it was in contact with the cork dissolve, deteriorate or damage the cork so as to break the seal??
freedom is absolute, I wish to be free
(Hive Bee)
03-13-03 00:08
No 416284
What SWIM would do is not to store it in a beaker but to store it in a PET plastics bottle with a lid .Why do you want to store it in a beaker with a cork ? .
Littlejase
" Better to be than not to Bee "
(Chief Bee)
03-13-03 00:12
No 416286
You should rather use a proper bottle with a chemical-resistant cap. PET is a polyester plastic which is slowly dissolved by GBL.
(Stranger)
03-13-03 00:25
No 416292
I am trying to determine if gbl affects simple materials such as cork for a hypothtical reason, thus really being able to use the correct materials for storage are not the issue. As we are all cunducting hypothetical exiperiments here anyways
The real question being would my theory about using the cork be acceptable for a short period such as 7 days?
(Hive Bee)
03-13-03 00:27
No 416293
Sorry Rhodium your right thats what SWIM sould do and if you don't have a proper bottle with a chemical-resistant cap then Swim would put it in a PET plastics bottle . But not a breaker with a cork .
Littlejase
" Better to be than not to Bee "
(Hive Bee)
03-18-03 09:52
No 418444
pardon my ignorance, but is polyethylene a polyester? that doesn't sound right to me. _P_oly_ET_hylene note the upper case bits. polyethylene is okay with alcohols but is slightly damaged by chlorinated solvents and aliphatics, and a lot damaged by ketones and aromatics. afaik.
worth looking at the info about materials/solvents compatibility that is either linked from here or rhodium. some materials just turn to goop with some solvents. like acetone and nylon... man.
It means buckle your seatbelts dorothy, because kansas ... is going bye bye
(Hive Addict)
03-18-03 12:06
No 418497
Polyethylene isn't an polyester,it's structure goes like
-CH2-CH2-CH2-
For those about to synth,we salute you
(Hive Bee)
03-18-03 20:07
No 418683
'ene' implies a double bond
what you drew would be called a linear alkane.
ethylene looks like this:
H
|
H-C=C-H
|
H
thus polyethylene would look like this:
H H H H
| | | |
H-C=C-C=C-C=C-C=C-H
| | | |
H H H H
though probably longer usually.
that doesn't come out with a monospaced font for me, is that how it is for everyone else?
how about this:
CH2=CH1-CH1=CH1-CH1=CH1-CH1=CH2
polyester would have to have oxygens and OH's in it just like an ester does.
edit: pardon me, I put too many hydrogens in previously, I've corrected this now. thanks badbody
It means buckle your seatbelts dorothy, because kansas ... is going bye bye
(Stranger)
03-19-03 04:01
No 418891
Ok so its ok on plastics but how about cork???
(Stranger)
03-19-03 07:02
No 418940
Normally you'd be right, urushibara. "ene" does normally imply double bond. In this case, however, ethylene is the monomer. The pi-bond in one ethylene molecule breaks and connects to the next one, forming a sigma-bond between them, which causes the pi-bond in the second to break, which joins to... and so on. The equation would be written:
x(H2C=CH2) --> ...-CH2-CH2-CH2-CH2-...
The name of a polymer like this tends to be written Poly-<insert name of monomer here>. For example, Poly Vinyl Chloride:
x(H2C=CHCl) --> ...-CH2-CHCl-CH2-CHCl-...
Or even Poly Tetrafluoroethylene (Teflon):
x(CF2-CF2) --> ...-CF2-CF2-CF2-CF2-...
Does that make it clear? I'd post a reaction mechanism (complete with curved arrows!) to make it clearer, but you can find them in an average text book, plus I have no idea of how to post pictures!
(Hive Bee)
03-19-03 08:18
No 418958
so polyethylene is a similar chemical in structure to hexane or dodecane or something then?
Is the plastic in naptha bottles polypropylene or polycarbonate? or does that vary?
It means buckle your seatbelts dorothy, because kansas ... is going bye bye
(Hive Addict)
03-19-03 12:53
No 419028
Yeah,i wondered too...but look what this document has to say about polyethylene
http://www.nrc.ca/irc/cbd/cbd154f.html
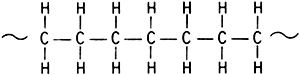
No double bonds...which made me wonder why it is called polyethylene...
Raf is no chemist by any means,i'll see what a chemist i know(swiraf's dad) has to say about that.
For those about to synth,we salute you
(Hive Addict)
03-19-03 12:57
No 419030
> No double bonds...which made me wonder why it is called polyethylene...
because it's polymerised ethylene (duh!)
i'd guess that there's side links to a varied
degree, giving PEs with different properties...
(Hive Addict)
03-19-03 13:34
No 419035
Why not polymethane?
For those about to synth,we salute you
(Chief Bee)
03-20-03 00:31
No 419188
Because it is made from ethene, not methane.
(Stranger)
03-20-03 10:19
No 419370
03-22-03 05:49
(Rated as: UTFSE!)
(Hive Bee)
03-22-03 17:15
No 420187
for a hell of a good time!
Its used as a solvent for cleaning electronic circuits, but most people I think people like to drink it more then they like like do clean stuff with it!
(Hive Addict)
03-22-03 17:26
No 420191
> _P_oly_ET_hylene note the upper case bits.
not really.
PE = polyethylene
PET = Polyethylenterephthalat
(but apparently PET is used for all kind of polyesters)
(Stranger)
03-26-03 02:47
No 421274
swim wants to know what it will do to cork--not pet or polyester or hdpe, Im just curious about cork---anyone see a trend in my question
(Hive Addict)
03-26-03 02:51
No 421276
Suberin (45%) - the main component of the cell walls; responsible for the resilience of the cork
Lignin (27%) - the binding compound
Polysaccharides (12%) - components of the cell walls which help define the texture of the cork
Tannins (6%) - polyphenolic compounds responsible for colour
Ceroids (5%) - hydrophobic compounds that ensure the imperviousness of cork
Mineral water, glycerine, and others make up the remaining 4%.
GBL can cause some deterioration and wear to the cork. Use glass vials with ground glass stoppers for maximum storage longevity.
Chemistry is hard to learn, but its worth it.
(Stranger)
03-30-03 06:41
No 422432
A true master of life---I truly thank you for your time and the amazing wisdom you have helped bring to us all---these are real praises--thank you soooo much for responding to the heart of the thread-- thank you thank you thank you-- I knew you couldnt resist a challenge
may the sun be upon your face
the wind at your back
and your glass always half full