07-05-03 20:44
No 444729
Raf acquired a pump lately,it seems to be only slightly used and 2 staged,550 watts,gas ballast and all.Raf was testing his pump,distilling pure safrole to be more precise,safrole started boiling at 60 C and temp constantly rose and finally stabilized at 108-110 which translates roughly to 10 torr vacuum.For some reason vacuum was falling throghout distillation process.Raf was wondering what would happen if he connected two of these pump in a row for more effiency?Sounds like a good idea,doesn't it?


For those about to synth,we salute you
(Chief Bee)
07-06-03 02:56
No 444795
A dry ice trap to protect the pump you already have would be a better investment, a likely reason for the falling vacuum was that as you distilled, volatile low-boiling terpenes from the oil dissolved in the vacuum pump oil, reducing the maximum attainable vacuum as more and more volatiles dissolved.
Osmium has posted a lot on this subject, go back and search for vacuum (pumps) and his name.
(Stranger)
07-06-03 06:31
No 444818
And also already by Osmium advised but seldom followed:
The use of metal tubing with at least double the diameter as the plastic tubing seen on the picture gives impressive better results. (the last meter to the flask is always flexible of course - use metal as far as possible) A aspirator as forepump and the ballast valve open max. helps to keep oil clean and is good enough to achieve a very good vacuum.
(Hive Addict)
07-06-03 08:34
No 444835
Oil wasn't probably the problem,he distilled safrole 5 times with it and every time boiling started at 60-70 and ended at 110 C.He should try to distill some isosafrole to get more precise results as distilling iso isn't as tricky as distilling plain safrole,will search for that Os post...
For those about to synth,we salute you
(Hive Addict)
07-06-03 09:27
No 444846
> The use of metal tubing with at least double the diameter as the plastic
> tubing seen on the picture gives impressive better results.
really? shouldn't this only become an issue, when the mean free path
length is approaching the diameter of the tubing? i.e. below 1mbar?
(Newbee)
07-06-03 12:20
No 444864
And below a certain vacuum it becomes essential. Gladly you added the <1mbar at the end - otherwise it would have been complete impossible to decrypt this piece of art. Oh? This is a sentence? Sorry....
In the range of vacuum raffike works the metal tubing is for sure measurable. The manufacturer of the pump didnīt attach this first class flange on his pump for to attach a gardenhose - a nibble would have sufficiced if this had been intended. Also in laboratories fixed installations are only used because they are more expensive, look better and make a better sound when you bang with the head against such a metal tube. The named "mean free path length" is probably measured in "meanometer" or "french" as unit? Just a guess....
(Hive Addict)
07-06-03 15:17
No 444874
Let me tell you what raf will do:he'll throw away that "garden hose" and replaces it with PP black plastic tubing,.5" in dia,keeps lenght minimal and tries how pump pulls,then he also tries connecting two identical pumps in row.Results to come...
For those about to synth,we salute you
(Newbee)
07-07-03 01:39
No 445016
5" (about 12cm, arnīt you european?) is to much volume added! will take to long to empty it and makes no sense you donīt have a turbomolecular highspeed pump on work
An aspirator would be better as a second pump - you have the dander to reach the vapour pressure of the oil of the first pump if two staged up. Might ruin it. A cooled trap with ATF (automatic transmission fluid) or the same oil as in the pump is easy and sucks exactly such vapours which would contamine the pumpoil. Clean it by boiling/heating.
I reread your first post and I believe it possible you have a nasty leak somewhere probably on some elastic part which blows open dureing use but kind of selfseals when vacuum is gone. Just a guess.
(Chief Bee)
07-07-03 02:35
No 445032
(Rated as: excellent)
Taken from Coyne's "The Laboratory Companion", Ch. 7 "Vacuum Systems", p 333-337, Wiley Interscience (1997)
7.2.7 How to Make (and Maintain) a Vacuum
Aristotle's statement "nature abhors a vacuum" means that even if you are successful in creating a vacuum, your ability to maintain that vacuum can require an equal, if not greater, amount of work. When creating a vacuum, you must establish your needs, define (and understand) your conditions, consult with authorities before you make purchases, and understand (and accept) any compromises.
We often euphemistically refer to creating a vacuum as emptying a container of its contents. But, what does "empty" mean? We already stated that it is impossible to make a container void of contents, so what do we need to do to "empty" a container?
There are three levels that should be considered when "emptying" a container. For example, consider emptying a glass of water: First you take the glass and pour out the water. From a simple aspect, the glass is empty. However, there is still a film of water in the glass, so you dry the walls of the glass with a towel until they are dry to the touch. However, if you want the glass really dry, you need to bake out the water that has adsorbed on the walls and is absorbed into the glass walls (water can soak into a glass matrix up to 50 molecules deep). Thus, when we talk about creating a vacuum, until you remove the adsorbed (surface concentration) and absorbed (material penetration) gases and vapors, you do not have an "empty" system.
So, say that you take a glass, pour out the contents, dry the walls, and bake out the glass so it is truly empty. The question is, Will it now remain empty until water is poured back in? As far as vacuum science is concerned, no. As soon as the glass is exposed to the atmosphere at room temperature, the walls will resaturate with water vapor, and the glass will no longer be empty. To maintain a glass as "empty", it must be isolated from the atmosphere. Otherwise you must repeat the drying process.
Unfortunately, glass vacuum systems cannot be "baked out" (as is done with metal vacuum systems) to remove adsorbed water. Baking is likely to damage stopcocks or rotary valves; or if the baking temperature is sufficiently high, the glass walls of the system itself. Thus, glass vacuum systems are not practical if baking your system is required.
What typically happens with a glass vacuum system is that first a mechanical pump removes a great deal of the "loose", or "free", gas particles. Then, greater vacuum is achieved with the combination of a diffusion pump (or similarly fastpumping unit) and traps that remove or bind up the various vapors within the system (for example, oil, mercury, and water). The only way a system can achieve a vacuum lower than 10-6 to 10-7 torr is if the pump can remove water vapor faster than the water vapor can leave the walls. Most diffusion pumping systems cannot achieve this goal, but even if they could, there is such a substantial amount of water vapor within the glass that, unless the walls are baked, a better vacuum cannot be obtained.
Aside from adsorbed gases, there are potential leaks in any vacuum system that must be dealt with or a viable vacuum cannot be created. Leaks and leak detection are dealt with in Sec. 7.6.
7.2.8 Gas Flow
As one is trying to remove the various gases from a vacuum system, whether the system is a bell jar or a complex collection of tubing with valves or stopcocks all interconnected, the gases must pass through tubing of various types and sizes between the system, the pumps, and the outside world. Depending on the size of the connecting tubing, the length of the tubing, and the vacuum at any time, there can be significant performance changes throughout the system. This all has to do with how gases flow through tubing of different sizes and at different pressures.
There are three basic types of gas flow: turbulent, viscous, and molecular. The type of flow passing through a given system is dependent on both the mean free path (MFP) of the molecule(s) and the diameter of the container (tube) through which they are flowing. A useful formula when talking about MFP is the Knudsen number (Kn), defined in Eq. (7.6).

When a system is first brought into vacuum conditions from atmospheric pressure, the flow is turbulent (see Fig. 7.3) and is not unlike the chaos seen in whitewater rapids. At this time, the MFP is approximately 9x10-7 cm, which is considerably smaller than any tube the gas is likely to pass through. The great preponderance of interactions (that is, when a gas atom or molecule interacts with something else) are gas-gas, which means there is a greater likelihood that a molecule of gas will hit another molecule of gas than it will hit a wall.
As the pressure decreases, the next type of flow transition is called viscous flow. The nature of this flow is complex and is dependent on flow velocity, mass density, and the viscosity of the gas. Viscous flow is similar to the flow of water running down a slow, calm stream-the flow is fastest through the center of the tube, while the sides show a slow flow and there is zero flow rate at the walls (see Fig. 7.4). The gas interactions in viscous flow are gas-gas and gas-wall; in other words, a molecule is equally likely to hit a wall than another molecule.
When Kn of the system is <0.01, the flow is probably viscous. The transition between viscous and molecular flow is fairly straightforward: When 1 > Kn > 0.01, the flow is in transition to molecular flow; when 1 < Kn, there is a molecular flow.
Fig. 7.5 When the mean free path is fairly short (relative to the inside diameter of its container), a gas molecule to more likely hit another gas molecule than the walls of the container. This situation is known as a gas-gas interaction.
Fig. 7.6 When the mean free path is longer than the diameter of the container of the gas, gas-wall interactions will predominate and you will have molecular flow.
Typically, the first phase of molecular flow is gas-gas. That is, a molecule or atom is more likely to interact with another molecule or atom than the wall (see Fig. 7.5). As the pressure continues to drop and the mean free path increases, gaswall interactions become the predominant type of gas flow.
One of the more interesting characteristics of gas-wall interactions is that not only are the gas reflections not specular (mirror-like), but the molecule or atom may go back into the direction that it once came from (see Fig. 7.6). This bounceback is partly because, at the molecular level, wall surfaces are not smooth but very irregular. In addition, there is likely to be a time delay from the time a molecule hits a wall to the time it leaves the same wall. At the molecular level, when a molecule hits a wall surface, instead of reflection (like a billiard ball), the process is more likely to be adsorption (condensation). When the molecule leaves a wall surface, the process is desorption (evaporation), thus explaining why there is the random movement and time delay for molecular reflection.
The gross movement of molecules in a high-vacuum state is a statistical summation of the parts. For example, say you have two containers that are opened to each other through a small passageway (see Fig. 7.7). One of them (A) is at 10-3 torr and the other (B) is at 10-5 torr. The movement of molecules in both is completely random, but the net movement will be from A to B. There will be molecules from B that will find their way into A, even though the pressure is greater in A. But again, the net molecular movement will be from A to B. Once the net pressure of 10-4 torr is achieved, there will still be movement between the two containers. Eventually, according to the first principle of gases presented earlier, there will be the same number of molecules in B as in A. There will always be a greater number of A molecules than B molecules, but the number of molecules on either side of the system will eventually be the same.
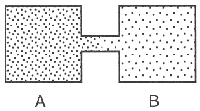
The time necessary for the molecules to travel from A to B (or B to A) depends on the abilities of molecules to pass through a tube. This passage is dependent on three things: the pressure difference between A and B, the diameter of the connecting tube, and the length of the connecting tube.
The Relation of Tube Length and Diameter to Pumping Time
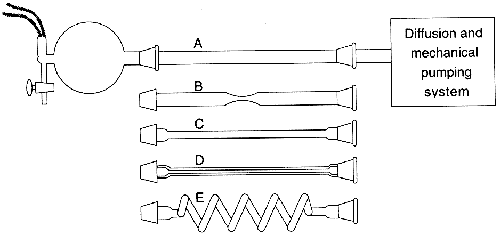
| Tube | Length (cm) | Bore (mm) | Time (sec) |
| Flask only, no tube | 14.1 | ||
| A | 50 | 20 | 21.5 |
| B | 50 | 20 with small section of 7.3 | 30.8 |
| C | 50 | 10 | 64 |
| D | 50 | 3 | 1800 |
| E | 150 | 3 | 10800 |
An interesting experiment was done by Barbour with a simple vacuum system of a 5-liter flask connected to a thermocouple with an opening for dry air. The flask was adapted to receive one of five different tubes (of different diameters and lengths) and in turn was connected to a pumping system (see figure). Barbour evacuated the 5-liter flask and then filled the system with dry air. He then re-evacuated the flask and calculated the time it took to go from 10-2 to 10-3 torr.
(Chief Bee)
07-07-03 02:36
No 445033
What's wrong with my table?
(Hive Bee)
07-07-03 03:36
No 445045
Pressures above about 10-2 mbar have viscous flow conditions and the conductance of tubing varies with the fourth power of its diameter: doubling tube diameter increases the conductance 16 times.
Conductance varies inversely to tube length: doubling the tube length halves the conductance
This means that tube diameter is much more important than length, though length should be reduced as far as possible.
(below 10-2 mbar, in molecular flow conditions, the conductance is proportional to the third power of the diameter and inversely proportional to length.)
Keep the tubing short and wide so that the performance bottle neck is with the conductance of the glassware and the pumping speed of the pump, which are fixed.
Typically tube conductance should be greater than 5 times pumping speed. I'd go for at least 20 mm internal diameter tubing.
HRH The Prince of Wales and Earl of Chester, Knight of the Garter, Rogerer of Servant Girls
(Hive Addict)
07-07-03 08:34
No 445138
> What's wrong with my table?
the first row has 3 cells, the second 5 cells and the rest
has 4 cells. they must all have the same # of cells.
(i think i just invented a new profession: table corrector
(Hive Bee)
07-07-03 15:24
No 445206
If nature abohrs a vacuum, why then does the universe employ one as the medium for the area between the stars and planets?
Many have stated: Working with a vacuum sucks, although, working with pressure blows. Working tables is what one does after getting a degree in fundemental geography, and not suprisingly, this is a prime example of a vacuum constant.
Use joint lube on glass glass joints,
Teflon tape on threaded connectors,
And when working with a vacuum, be aware that some labware is not neg.pressure friendly, and thus will implode at the worst possible moment.
Try to eliminate as many connections as is feasible, and also test the vac. pump to spec.s to assure that it is a sound unit at all levels of vacuum inducement.
Karma? Karma is on Murphy's payroll. Nothing improper, it's freelance.
(Hive Addict)
07-07-03 21:07
No 445284
Isomerized some safrole(230 C and 3 hours,only 55% yield,will switch to vac isomerizing soon) and tried my pump again this time with 20 mm hose which was very short,same results,120 C was the bp of isosafrole which means it pulls as much as last time with safrole(safrole came over at (108-110).Will try 2 pumps in a row soon.
For those about to synth,we salute you
(Hive Bee)
07-08-03 06:10
No 445447
Raffike, what condition is your pump oil? Did it come with the pump or is it likely to have been changed? An oil change is the first thing I would do with a used pump.
HRH The Prince of Wales and Earl of Chester, Knight of the Garter, Rogerer of Servant Girls
(Hive Addict)
07-22-03 00:39
No 448902
Raff, brand new yellow jackets cost $300bucks and boil sassy ar 55c. I don't see why people keep knocking them. I bought one of mine used from a retired refrigeration technician and he never changed the oil the entire time he owned it and it sat in his garage for ten years with that same oil init I changed the oil and ran it a while and changed it again and it runs sassy at 65c. I assume it operates as it did new the newer ritchie has a lower ultimate vacuum rating thatn that old ass one.
Yes, That pic really is me!
(Hive Addict)
07-22-03 08:53
No 448993
Got that one free.Had to buy a kwt motor but that was 3 bucks for me.I'll see if i can get a yellowjacket somewhere.
For those about to synth,we salute you
(Hive Addict)
07-22-03 09:33
No 449008
(Rated as: good idea!)
is the original problem solved already?
a possible (likely) explanation of vacuum getting worse during
distillation is oil impurities:
pump temperature rises -> vapor pressure of impurities increases -> attainable vacuum gets worse.
(Hive Adickt)
07-23-03 03:53
No 449128
I said it before.
Use a freeztrap (those 10$ on dry ice is spent well.)
Iff (for some reason ) you don't use a freeztrap, let the balast bee at max, after the destilation let the pump run (with a stoppeer in the tube) for 1-2 houer, this blow most of the polutan's out of the oil. A wac.pump. is a delicat instument. Treatet well, it last for a lifetime. Treatet bad it last 6 month.
(Hive Addict)
07-23-03 03:58
No 449130
His vacuum drifts from 60-150c?? Sounds more like bad internal components of the pump. You should look into a rebuild kit for your pump. Or call the manufacturer and see what they reccomend...
I see your point hest But my pump sucks more vapours then his does and when distilling safrole here in texas at 90f room temp and 55c boiling temp evaporation form the recieving flask has to be maximal and I doubt he is getting enough tgoods in his pump to affect it that wildly. Just by looking at the pic and comparing it to others I have see like it with metal flanges his shold outperform a yellow jacket any day. And I have run lond distillations of several liters with supposedly dirty oil from previous procedures and the most my temp ever drifted wat 5c that was mostly due to the strong vacuum sucking my grease out of my joints and causing leakes which was fixed using teflon sleeves. I just don't see that big a pump drifting that far from a lil imnpurities I'd say its more from mechanical wear or both. The impurity content would have a bigger impact on a worn pump. Just my two bits I may be wrong but jsut think about it.
Yes, That pic really is me!
(Hive Bee)
07-23-03 11:14
No 449216
No, I donīt think raffike is to stupid to get the right motor but e-motors are buildt in very different ways and have very different characteristics. Perhaps the pump runs to fast - also if the named data on motor and pump look right. Try running slower by an controller or another motor - a controller would be preferred for giving a very nice control over the pump in addition (and is usable for most pumps and else stuff...)
Running to slow by rising/decreasing load is also possible but less probable btw.
(Hive Addict)
07-23-03 11:34
No 449222
Pump was bought used and was returned.He'll buy a new one to be sure.It's not very expensive...
For those about to synth,we salute you