07-16-01 19:27
No 190955
This is from an old Russian chemistry textbook:
Alkylation with alkyl esters of arylsulfonic acids:
C6H5ONa + ArSO2*CH3O ----> C6H5OCH3 + ArSO2*Ona
The rxn is carried out just like with dimethylsulfate, by energetic shaking the mixtr of the reagents in aq. alkali, followed by heating. It also goes to completion in alcoholic soln or by heating the dry phenolate with addition of a neutral solvent where necessary (benzene, abs. acetone)
It is noted there that these esters are by far not as poisonous as dimethylsulfate is, which is surely nice, I must say. However, they still are “partially toxic” and tend to cause exema (I don’t know how it spells, yes…)
The esters themselves (mainly p-toluylsulfonic methyl ester), the book states, can be prepared from mixing toluylsulfochloride w/methanol, followed by aq. NaOH with cooling below 25 C. Its mp is 28 C, mp of ethylester 32-33 C.
The book also says that toluylsulfochloride is easily available as a by-product of sugar industry. But for someone i have known and loved, PCl5 or POCl3 to make it are out of question because of their being grossly expensive (and so is dimethylsulfate).
So he wonders, if it is possible to make them in a conventional manner that acid esters are made – that is, by simply boiling p-toluylsulfonic acid w/methanol with some catalytic H2SO4?
P- toluylsulfonic acid can be easily made by boiling toluene w/H2SO4 conc.
Humbly awaiting your replies,
Antoncho
P.S. This information wasn’t exactly found by me, it comes from a Russian small analog of the Hive, named HyperLab (www.hyperlab.org or http://www.voy.com/12987). This place – the Hive, I mean – has always been the main source of data input for this petite clandestine community
(Chief Bee)
07-16-01 19:42
No 190962
If the esterification is possible that way (which I think it is), the catalytic H2SO4 is definitely not needed, the toluenesulfonic acid is good enough as catalyst.
If I take a look at the board, it is almost exclusively in russian, which makes it impossible for the rest of us to understand (afaik, you are the only russian on this board), so could you please copy over the most interesting syntheses to this board? Also, is there anything revolutionary to be found at http://dissociative.h1.ru/ ?
I would also like to say that you are invaluable for us here at the Hive, as you can translate all those interesting articles and patents we find.
(Hive Addict)
07-16-01 21:33
No 190984
in terms of methylating something, would this apply to mono-methylation of amphet? methylation of OH on ring to methoxy? what about aliphatic OH's?
(Chief Bee)
07-16-01 21:46
No 190987
It would apply to phenols and possibly aliphatic alcohols. Methylating amphetamine with it would produce a mixture of mono- and dimethylated amphetamine.
The Hive - Clandestine Chemists Without Borders
(Stoni's sexual toy)
07-17-01 00:07
No 191016
Another good methylating agent is trimethlyphosphate, and I heard rumours that dimethylcarbonate works too.
(Official Hive Translator)
07-17-01 10:55
No 191149
If the esterification is possible that way (which I think it is), the catalytic H2SO4 is definitely not needed, the toluenesulfonic acid is good enough as catalyst.
Thank you very much for your comments, Rhodium! I guess i'll try this rxn over the weekend and post the results, however i have nothing methylatable at hand to test it upon. Which brings me to ask you, guys, how would one go about separating the ester from the unreacted acid? I'd imagine that methyl toluylsulfonate is pretty soluble in water, isn't it?
Can you just saturate the mixtr with aq. bicarbonate and extract the ester w/a non-polar? I'm sorry to ask such basic questions but i don't really have much practical expeience w/lab chemistry...![]()
![]()
If I take a look at the board, it is almost exclusively in russian...
yes, it is...![]()
afaik, you are the only russian on this board ...
As far as i know there used to be another Russian guy here - that one was a real chemist! - named Assholium. The one that claimed to have made the 4-rhodano-2,5-methoxy thing (which i have no doubt he indeed had). He said there were still many syntheses by him on your page :):) But we also lost contact w/him a long while ago. You might also be familiar we someone by nickname Jerry (aka Akim D. aka Apologet) who was the founder of our Russian project; as far as i know, he had a few acquaitances both here & in Lycaeum.
By the way, what does 'afaik' mean?![]()
so could you please copy over the most interesting syntheses to this board? Also, is there anything revolutionary to be found at http://dissociative.h1.ru/ ?
Yes, i'm working on it... Not like there's an awful lot of new information, but a couple of synths will surely surprize you...
http://dissociative.h1.ru/ deals mainly w/syntheses of PCP and its analogues - it used to be very popular in European Russia - , i believe that most methods on the site are not covered here, but i didn't get into much detail when looking through them, being uninterested.
I would also like to say that you are invaluable for us here at the Hive...
Oh my fucken Jesus... no comments, though ![]()
![]()
![]() . Always welcome.
. Always welcome.
Antoncho
P.S. Can anyone, PLEASE, take a look at my write-up about Al+NaOH+CH3OH in Acquisition forum? Really need some clarification on the subject.
(Official Hive Translator)
07-17-01 10:58
No 191150
I noticed the change in the title... Dear Sirs, let me gratefully tell you that I feel truly hohored...
Antoncho
(Chief Bee)
07-17-01 13:15
No 191157
Synthetic suggestion:
Boil 100g of p-toluenesulfonic acid with 1000ml methanol for 12h, evaporate most of the methanol under vacuum, wash with 50-75ml portions of concentrated aqueous bicarbonate until neutral (backwash the bicarbonate extracts with 2x50ml non-polar), add an equal amount of water and separate the organic layer. Extract the aqueous phase with 3x50ml non-polar solvent. Combine all the non-polar extracts and remove the solvent under vacuum, and if desired (I think it is needed) vacuum distill the methyl p-toluensulfonate.
Afaik stands for "as far as I know"...
The Hive - Clandestine Chemists Without Borders
(Stoni's sexual toy)
07-17-01 15:10
No 191170
Didn't he use Br2/thiocyanate? Or will that mixture form the dimer of thiocyanate?
(Chief Bee)
07-17-01 16:03
No 191176
Yes, he did. The literature does not clearly answer if 2 KSCN + Br2 produces KBr + BrSCN or 2 KBr + (SCN)2, so the active thiocyanation agent may either be BrSCN or (SCN)2 in his case. Either way, a test run on 1,4-dimetoxybenzene yielded only starting material and an orange thiocyanate polymer. Pb(SCN)2 + Br2 (which gives PbBr2 + (SCN)2) in solution also gave nothing but starting material, but less polymer was formed. A literature search confirmed that thiocyanation of phenol ethers requires a lewis acid catalyst, preferably Al(SCN)3 (but this has not been tested).
(Official Hive Translator)
07-28-01 20:42
No 199573
Well i'm afraid this rxn doesn't work at all
I still post the story of what SWIM did so that you guys (Rhodium?
p-Toluenesulfonic (tosic) acid (method taken from http://zaratustra.narod.ru)
In a 500 RBF equipped w/a reflux condenser SWIM placed 76 ml toluene and 45 ml conc. H2SO4. They didn't mix, duhh. This was immersed in an oil bath which was maintained at ~125 C during the rxn. The toluene never really started to boil, so SWIM just shook it occasionaly for an hour (don't take the condenser off! Toluene starts to boil as you shake it) - maybe 12 shakings were needed. At this point most toluene was consumed (26 ml left) and the bottom layer was brownish yellow. To this 170 ml of water and 1.7 g activated charcoal were added and some of the mxtr was distilled off until the distillate stopped smelling like toluene. The charcoal was filtered off at this point and the mxtr became clear and slightly green. It was further concentrated to 140 ml volume.
SWIM then put it in the freezer overnight and the next morning a little amt of crystals was observed at the bottom. The flask was immersed in an ice bath and saturated w/unhydrous HCl at 5-10 C. (Jesus, you should've seen SWIM's kitchen lab! All the equipment w/the xception of the (the only:) condenser is self-made
But SWIM , being young and impatient, decided that since water is formed in the rxn anyway and HCl would very soon go away as CH3Cl (as indeed happened) he'd just take some of his wet tosic acid and proceed w/it. So he weighed out 22 g of it (2 g added for moisture) of it, dissolved in 100 ml CH3OH and dryed the soln. w/freshly pulverized Na2SO4 - an large excess was added and left in the flask to absorb the moisture that would supposedly be formed further. This was refluxed for 18 hours. The mixtr turned yellow and unclear. It was neutralized w/saturated aq. bicarbonate (~130 ml) (CO2 evolution was observed) and xtracted w/ 2x70 ml ethyl acetate, which removed all yellow color to the org. layer. The organic xtracts were separated and dryed w/Na2SO4.
Now SWIM doesn't have a vacuum so he just decided to boil the solv. off at ordinary pressure (ethylacetate bp 77 C, methyl tosylate bp 140 at 5mm) (bath set at 100 C), and when he did that all that was left in the flask was ~0.5 ml of dark brown gunk. Now, SWIM thought that maybe since they're both esters they might form an azeotrope or smth (SWIM doesn't know shit in chemistry;) and tried to evaporate the ethyl acetate from the distillate w/a hair dryer (a vacuum cleaner attached to the system as the fume hood
Well, ladies and gentlemen, looks like this approach doesn't work.
FUCK!!!
Well, maybe that methyl tosylate is so volatile that SWIM evap'd it all away but i highly doubt that
Still tosic acid is a useful reagent - it can be used in isomerizing THC. Also SWIM plans to try it as a TFA substitute in Duff formylation of dimethoxybenzene - that'd be really cool! If it worked...... SWIM is starting to get pessimistic - too bad for him!
BTW, can anyone w/an access to the library look up its pKa? I shall be REALLY grateful as i can't find it on the Web.
Antoncho
(Hive Addict)
07-29-01 03:44
No 199648
Be carefull in that all methylating agents - dimethylsulfate, methyl iodide, methyl bromide and probably even methyl toluenesulfonate are toxic and possible carcinogens.
Anyone know the chemical name of the methylating agent sometimes referred to as "Magic Methyl"?
(Chief Bee)
07-29-01 05:37
No 199671
Methyl fluorosulfonate.
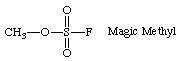
../rhodium
(Hive Addict)
07-29-01 19:02
No 199791
is the flourine what causes that Me to come off so easily?
(Chief Bee)
07-29-01 19:28
No 199793
Yes, as the fluorine is so electron-withdrawing, it stabilizes the SO3F- anion which forms when the methyl group comes off to such a degree that the compound will methylate just about anything.
(Hive Addict)
07-29-01 21:22
No 199801
what kind of availability and cost is this compound?
(Chief Bee)
07-29-01 21:29
No 199804
The first hit on google for the compound is selling it for about $150 for 5g. I'd say that it is expensive
../rhodium
(Hive Addict)
07-29-01 22:04
No 199812
$150 for 5g
Probably mostly handling charge. I seem to remember hearing a story of someone dying after spilling just a drop on the sleeve of his labcoat.
(Hive Addict)
01-24-02 23:43
No 260477
Is there any other route to separating the p-ToS? The gassing is something I'd really like to avoid.
(Official Hive Translator)
01-25-02 03:22
No 260561
Yes, with conc. HCl - but the yields drop sharply in this case (the article says).
Antoncho
(Hive Addict)
01-25-02 05:48
No 260601
I've read a patent and scaled everything accordingly, does this seem OK:
32.8g 94% sulfuric acid
10.0g toluene
reflux at 115C, until toluene reacts (apx how long? couple hours?), cool to room temp
add 2.5g water down the condenser
filter off ~11.3g toluene-4-sulfonic acid
Will the H2SO4 eat through standard filter paper? Is there anything I can use to wash the crystals? Btw, will some MeOH in the toluene form the methylating ester that you desired?
(Official Hive Translator)
01-25-02 08:28
No 260666
>I've read a patent
Which one? Here's the source article for my experiment (http://moonlight.ionichost.com/toluenes
>reflux at 115C,
not really reflux, just near boiling/barely boiling - cause when you shake it it's gonna boil violently.
>until toluene reacts (apx how long? couple hours?)
yep. You'll see when it stops.
>add 2.5g water down the condenser
Whut???
>filter off ~11.3g toluene-4-sulfonic acid
no way.
>Will the H2SO4 eat through standard filter paper?
it definitely will, in no time
>Is there anything I can use to wash the crystals?
I dunno. If you do everything like in the article, they'll bee already quite pure.
>Btw, will some MeOH in the toluene form the methylating ester that you desired?
Seemingly, no, as SWIM had no luck doing it w/methanol. Ah, everything may bee - like he just did smth wrong or had wet methanol - SWIM was unforgivingly careless at the time, now life has taught him some precision
Antoncho