01-19-02 13:16
No 258415
I have seen references of benzyl sodium, is there any other names for this compound ?
C6H5.CH2Na I would assume the formula is
One day I'll understand everything !
(Ubiquitous Precursor Medal Winner)
01-20-02 03:00
No 258628
Sodium toluide. The preparations I have seen, react sodium with benzyl chloride. But one textbook ref said something like "the alkyl hydrogen of toluene is sufficiently acidic to react with sodium". Since I read that, I've been wanting to test my interpretation of that phrase, by refluxing toluene over Na metal to see if it dissolves. The advantage of that would bee in saving the half of your hard-won sodium which goes into making salt. Thus it might hypothetically need only one molar equivalent, instead of two as when using benzyl chloride. Probably just wishful thinking again.
turning science fact into <<science fiction>>
(Hive Bee)
01-20-02 04:20
No 258635
If lithium reacted in the same way as sodium to the chloride or straight toluene, then that would be interesting with regards to producing phenylacetone !
One day I'll understand everything !
(Hive Bee)
01-20-02 23:45
No 258901
Aryl Alkali-Metal Compounds (Organic Chemistry - Paul Karrer 1947)
Aryl compounds of the alkali-metals are formed with unexpected readiness by the action of the alkali metals on chloro-substituted aromatic compounds in the presence of an indifferent solvent, at a temperature not greater than about 40c. The aryl alkali-metal compounds produced can be used for further reactions without isolating them, or they can be produced in the presence of suitable reagents which combine with them as they are formed. They react with carbon dioxide with the formation of carboxylic acids; with benzonitrile they give ketones, e.g., benzophenone:
C6H5Na + NCC6H5 > C6H5C(=NNa)C6H5 >H2O> C6H5COC6H5
With sulphur dioxide they give sulphinic acids and with acetic anhydride they form mixed aromatic-aliphatic ketones. These compounds are therefore important starting substances for synthesis, resembling in this respect the alkyl magnesium salts.
Organic alkali-metal compounds of mixed aromatic-aliphatic hydrocarbons are also known. Sodium benzyl, C6H5CH2Na, and sodium triphenylmethyl, (C6H5)3CNa, are specially interesting on account of their red colour, and the fact that the sodium is linked ionically with the hydrocarbon radical.
...................
Lithium Alkyls may also be used in the synthesis of ketones. With dry carbon dioxide they react according to the scheme:
2RLi + CO2 > RCOOLi + RLi > RCR >H20> RCOR + 2LiOH
/ \
OLi OLi
giving di-lithium salts of the ketone hydrates, which break down on addition of water into ketones and lithium hydroxide (H. Gilman).
One day I'll understand everything !
(Master Whacker)
01-21-02 13:47
No 259119
halfapint:
When swim was younger and dumber, he actually used sodium to dry toluene and I can assure you no sodium reacts with the toluene at reflux temperature. This was before he realized simple distillation dries toluene azeotropically and as effeciently as Na.
(Ubiquitous Precursor Medal Winner)
01-22-02 13:57
No 259515
OK, good. Benzyl chloride wants to bee made, in a quartz tube of toluene, running up by the Hg vapor (short-wave UV) lamp quartz tube, when chlorine bubbles through the toluene, or so my buddy SWIM sez. None of the other easy ways give enough equipment challenge for her.
In a similar thread, I mentioned benzyl sodium is spontaneously flammable in air. Something like that, is worth mentioning again.
turning science fact into <<science fiction>>
(Hive Bee)
01-22-02 13:58
No 259517
This link suggests that only one Na is needed to make benzyl sodium from benzyl chloride,
http://jeandenoir.free.fr/content/folder
Or maybe it means that Na is added to toluene then it is gassed with chlorine.
One day I'll understand everything !
(Ubiquitous Precursor Medal Winner)
01-22-02 14:32
No 259547
A rough and ready translation:
Organoalkalis are prepared by action of an organic halide on the metal itself. This is how one prepares butyllithium:
C4H9Br + 2 Li ---> C4H9Li + LiBr
One generally works in ether. The presence of water must be excluded, and other protonic compounds which may be decomposed by organo-alkalis. One may also substitute an acidic hydrogen of a hydrocarbon for the metal of an organo-alkali without acid. It is thus that benzyl sodium is prepared:
C6H5-CH3 + R-Na ---> C6H5-CH2Na + RH
Organosodium and organolithium compounds are used as polymerization catalysts for conjugated dienes.
Looks like if ethyl sodium is refluxed in toluene, ethane goes away, driving things to the right. (Ultimately, you still use two sodiums, for your ethyl sodium was prepared from ethyl bromide using the first reaction.)
turning science fact into <<science fiction>>
(Hive Bee)
01-22-02 14:36
No 259548
C6H5-CH3 + R-Na ---> C6H5-CH2Na + RH
So whats the R representing ? a halide ?
One day I'll understand everything !
(Chief Bee)
01-22-02 14:48
No 259555
No, an alkyl chain, like ethyl or butyl. Butyl-Na is probably made from BuCl and Na, just like BuLi is made from BuCl and Li.
(Ubiquitous Precursor Medal Winner)
01-22-02 15:54
No 259571
You could use only one sodium, if the R- were acetylene. Sodium acetylide, unlike the heavy-metal acetylides, is not a boomer. Acetylene unites directly with molten sodium.
Does anyone know offhand, the thermal beehavior of a flask containing benzyl sodium in toluene, if it were dripped with acetyl chloride in toluene? How much would you have to chill it?
turning science fact into <<science fiction>>
(Hive Bee)
01-22-02 16:56
No 259612
It seems however the benzyl sodium is made, it is still a difficult reagent to deal with in a non-professional environment.
One day I'll understand everything !
(Ubiquitous Precursor Medal Winner)
01-22-02 17:15
No 259623
Probably won't burst into flame if it's in solution. But SWIM sez, just the same, any of her flasks that might have sodium alkyls in them are gonna bee covered with reducing gas that whole time...
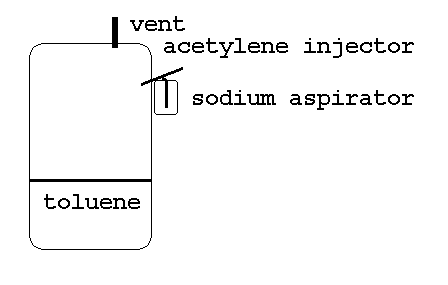
Hoping the fine droplets of sodium react with acetylene readily, in flight or hitting the side of the vessel, or the acetylene will have to get heated first. (Not much, don't heat acetylene too much.) The sodium acetylide falls into the toluene, where it gives off its acetylene. Benzyl sodium accumulates in the toluene.
turning science fact into <<science fiction>>
(Ubiquitous Precursor Medal Winner)
01-24-02 06:19
No 260327
From clues I've gathered around here, an acetyl ester will react with benzyl sodium, similarly to acetyl chloride, to produce P2P. The way I understand it, valuable by-products (for use in other reactions) such as sodium methoxide or sodium ethoxide might bee produced in this process. So not only can you use just one sodium, perhaps you can make it do double duty!
turning science fact into <<science fiction>>