03-10-02 05:49
No 279938
Why not? I don't understand why the Ritter Reaction "does not work" for methylenedioxy-amphetamines?
Does it fail for all substituted benzenes? How come? I does not understands...
PrimoPyro
Vivent Longtemps La Ruche!
(Chief Bee)
03-10-02 12:14
No 280053
(Rated as: excellent)
The Ritter Reaction involves the use of 50/50 acetonitrile:sulfuric acid as reaction medium, which turns added allylbenzene into 3-phenylpropyl hydrogen sulfate, which via a carbocationic intermediate adds across an acetnitrile molecule, and under the extremely acidic conditions the alkyl chain add to nitrogen and the sulfate to the carbon, forming an oximidic acid sulfate, which upon dilution of the reaction mixture with water forms the corresponding oximidic acid, which quickly tautomerizes to N-acetyl-amphetamine.
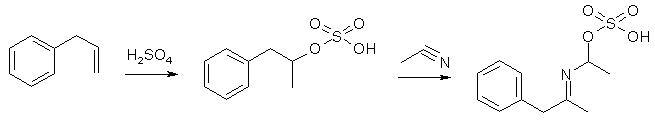
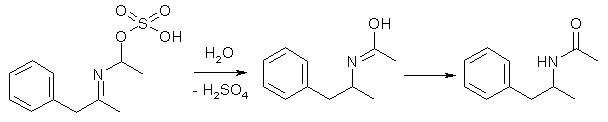
The conditions used - hot 50/50 acetonitrile:sulfuric acid - does not allow the reaction on any substrate with acid-sensitive functionalities like a methylenedioxy ring. A low yield may perhaps be obtained with methoxybenzenes, but the ritter reaction fails with safrole1, and the major isolable product after the reaction is 3,4-dihydro-1,3-dimethyl-6,7-methylenedi

[1] J. Am. Chem. Soc. 74, 763 (1952) (../rhodium/pdf /ritter.react
[2] Journal of Clandestine Laboratory Investigating Chemists Association (../rhodium /clic.h