03-25-02 15:16
No 287629
Quoted from March's Advanced Organic Chemistry, 5th Ed., p.1156, 19-46: Reduction of Miscellaneous Nitrogen Compounds
"A cyano group can be reduced to a methyl group by treatment with a terpene such as limonene (which acts as a reducing agent) in the presence of palladium-charcoal. H2 is also effective, though higher temperatures are required. The R group may be alkyl or aryl."
Kindler, K.; Luhrs, K. Chem. Ber., 1966, 99, 227; Liebigs Ann. Chem., 1967, 707, 26
Well if this were applied to the nitrile of phenylalanine, PhCH2-CH(NH2)-CN, then the nitrile would be reduced to a methyl group, forming PhCH2-CH(NH2)-CH3, which is of course amphetamine. How to make the nitrile?
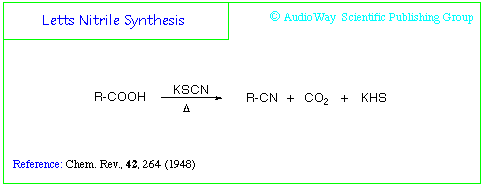
So if one were to use potassium thiocyanate with phenylalanine, forming the nitrile, one could then reduce the nitrile to a methyl with a terpene and Pd/C.
PrimoPyro
Vivent Longtemps La Ruche!
(Official Hive Translator)
03-25-02 16:40
No 287644
This is simply W_O_W !
Afsolutely bucking awesome!!
This Letts synthesis alone is worth more than a three thumbs-up! Say, what of applying this to a subst'ed phenylacetic acid? (PolytheneSam, you're reading this?)
If only some one could give us more precise data on this synth
Antoncho
(Newbee)
03-25-02 17:02
No 287646
SWIM as professional pessimist higly doubts that this will work. Look at link:
http://themerckindex.chemfinder.com/TheM
233.
Letts Nitrile Synthesis
E. A. Letts, Ber. 5, 669 (1872).
Formation of nitriles by heating aromatic carboxylic acids with metal thiocyanates
These old funny reactions almost always are fairly limited in scope.
(Stranger)
03-25-02 21:24
No 287734
what happens to the very active amine group , how is ist protected? Or since its more reactive than the carboxyl end why not methylate it first then go one to the carboxyl site with the conversion to..........what you stated. Just a thought after reading Mc Murry's text on the chemistry of amino acids.
Note :the pK a of COOH( -)=2.6 and the pKa of NH3(+)=9.2 so the amine aide will have a greater affinity to react . At least that's the way I read it.
(Hive Bee)
03-26-02 10:23
No 288032
Great! But I think for most bees its more easy to get 1kg amphetamine than 1g potassium thiocyanate
(Hive Prodigy)
03-26-02 10:25
No 288033
Why would that be? I can go buy it from the photo supply down the street! Its $22 per 100g.
Where can I get a kilo of amphetamine for $22?
PrimoPyro
Vivent Longtemps La Ruche!
(Chief Bee)
03-26-02 10:31
No 288036
Just a little tidbit: The Pd/C and limonene forms a classic catalytic transfer hydrogenation (CTH) system, where the limonene gives up hydrogen atoms to the nitrile. I bet other hydrogen donors like cyclohexene, ammonium formate or hydrazine would work too.
Don't give up on the nitrile synthesis just because the hyper-simple Lett's reaction doesn't seem suitable - there are a dozen other ways to make nitriles.
(Hive Prodigy)
03-26-02 10:35
No 288037
Rhodium: I thought that when nitriles were reduced with CTH, they formed amines?
Also, I am about to type up an additional question in my general chem question thread. Id much appreciate it if you would have a look at it in a minute.
PrimoPyro
Vivent Longtemps La Ruche!
(Chief Bee)
03-26-02 11:15
No 288049
Yes, usually they do. But what would treatment with limonene/Pd-C be if not a CTH? I guess the products are simply dependent on the reaction conditions.
(Hive Prodigy)
03-26-02 11:21
No 288051
I agree that by definition it is a catalytic transfer hydrogenation, I just made that one distinction is all.
I wonder, could the cycloalkene be part of the same molecule as the nitrile?
For instance, 1-cyclohexenyl-2-amino-propionitrile being self reduced and dehydrogenated to amphetamine.
PrimoPyro
Vivent Longtemps La Ruche!
(Chief Bee)
03-26-02 11:39
No 288058
In theory, yes, but one always need an excess of the hydrogen donor.
(Pioneer Researcher)
03-26-02 13:45
No 288081
CTH is super interesing reducing procedure, and I've seen in some papers that acoprding to the condicions can redice nitros selectivily without affecting for example nitriles, but it seems that there are CTH procedure to reduce nitriles to amines as well, and other to reduce oximes, I believe that the Hive should study more this kind of rxns. Could I suggest the refernce reseatcher to find and post all the CTH reductions possible ?
(Hive Bee)
03-27-02 06:03
No 288408
Hey PrimoPyro, where'd you get the idea for that rxn? aurelius posted something on this same general area a while back. actually, aurelius still has an article from JACS or JOCS or something like that on this.
(Hive Bee)
03-27-02 06:48
No 288414
It needs to be taken into accound that this is an Amino Acid. A R-COOH with and alpha-NH2 dos not react like a normal aliphatic carboxylic acid. In fact, it is a neutral species existing >95% of the time as the zwitterion. i.e., -CH(COO-)N+H3 .
The acid group is not acidic, and the amine is not basic. The way you usually get the carboxylic acid to act like an acid is to deactivate the Amine as a carbamate. Usually FMOC or BOC. And the way to get the amine to behave like an amine is to make the ester of the -COOH.
(Hive Bee)
12-02-02 17:51
No 385526
Slappy: You are right about the protecting group, but one can make the alcohol without it , hence "a mixture of NaBH4 and I2 has been used to reduce amino acids to amino alcohols" see Mckennon, M.J.;Meyers, A.I.;Drauz, K.; Schwarm.M.J.Org. chem., 1993,58,3568
Primo : page refrence on your post should be 1556 on your March's refrence.
Note: SWIJ efforts are to make honey from Phenylalanine so I'm always looking. So with that said (see )"Carboxylic acids can also be converted to alkanes , indirectly , by reduction of the corresponding tosylhydrazides (RCONHNH2) with LiALH4 or Borane" quoted from Marche's 5th edition page 1552.
Note: on this procedure one will have to use a protecting group for the amine since we want it to behave as an acid.
Ref:
Degani,I.;Fochi,R.J. Chem.Soc.,Perkin Trans. 1,1978,1133.
for a direct methos , (see) Le Deit, H.;Cron, S.;Le Corre, M. Terrahedron Lett.,1991, 32, 2759
Attanasi, O.;coglioti, L.; Gasparrini, F.; Misiti, D. Tetrahedron, 1975, 31,341,and cited refrences
Note :see../rhodium /amp
Saludos (Greetings), from Latin America