04-14-02 23:40
No 296918
When PdCl2 is in aqueous or alcoholic solution, it has four chloride ligands, provided they are available. This is how the wacker works. I have a book on oxidations using palladium, and it describes the entire method of the oxidation. The reactive complex is formed by displacement of one of the four chloride ligands with the alkene pi system. Then, another chloride ligand is displaced by a solvent molecule, followed by deprotonation of the solvent molecule (alcohol or water). This complex then rearranges to a sigma system by a cis transformation of the ligands and attack of the deprotonated solvent molecule on one of the two carbons. The palladium, which is now bonded to the opposite to the attacked carbon, abstracts a hydride from it and converts to a pi system. This pi system then reverts to a sigma system and the reduced palladium breaks off, transfering a hydride.
Cl Cl Cl Cl
\ / C \ /
Pd + \\ => Pd + Cl-
/ \ C / \ C
Cl Cl Cl //
C
Cl Cl ROH Cl
\ / \ /
Pd + ROH => Pd + Cl-
/ \ C / \ C
Cl // Cl //
C C
ROH Cl RO Cl
\ / \ /
Pd + ROH => Pd + ROH2+
/ \ C / \ C
Cl // Cl //
C C
RO Cl Cl Cl
\ / \ /
Pd => Pd
/ \ C / \ C
Cl // RO //
C C
Cl Cl Cl HOR
\ / \ /
Pd + ROH => Pd OR
/ \ C / \ /
RO // Cl C-C
C
Cl HOR Cl H
\ / \ /
Pd OR => Pd
/ \ / / \ C-OR
Cl C-C Cl //
C
Cl H Cl H
\ / \ /
Pd + 2ROH => Pd OR + ROH2+
/ \ C-OR / \ /
Cl // Cl C-C
C \
OR
Cl H OR
\ / /
Pd OR => PdCl2+2 + C-C
/ \ / \
Cl C-C OR
\
OR
(Pioneer Researcher)
04-15-02 01:33
No 296942
Thank you for the info.
(Distinctive Doe)
04-15-02 13:59
No 297169
"I have a book on oxidations using palladium"
Oh thanks for telling us what book is.......
Give your references ALWAYS
Do we need to ask you everytime you post something new???
I won't even ask why this isn't in the other Wacker Thread.
Foxy
Those who give up essential liberties for temporary safety deserve neither liberty nor safety
(Stranger)
04-15-02 16:39
No 297182
Palladium catalyzed oxidation of hydrocarbons
Henry, Patrick Mark
Sorry I don't have a photographic memory and can remember titles of books off the top of my head.
(Chief Bee)
08-22-03 00:55
No 454912
(Rated as: excellent)
"Palladium in Organic Synthesis"
Chapter 8, Nucleophilic and Electrophilic Addition and Abstraction
8.2 The Wacker Process
Alkene complexes undergo nucleophilic attack to give metal alkyls, which can often rearrange to give other products. This is the basis of an important industrial process, the Wacker process, now used to make about 4 million tons a year of aldehydes from alkenes. The fact that aqueous PdCl2 oxidizes ethylene to acetaldehyde had been known1 (although not understood) since the nineteenth century; the reaction consumes the PdCl2 and deposits metallic Pd(0). It took considerable imagination to see that such a reaction might be useful on the industrial scale, because PdCl2 is far too expensive to use as a stoichiometric reagent in the synthesis. The key is catalysis, which allows the Pd to be recycled almost indefinitely. J. Smidt2 of Wacker Chemie realized in the late 1950s that it is possible to intercept the Pd(0) before it has a chance to precipitate, by using CuCl2, which reoxidizes the palladium and is itself reduced to cuprous chloride. This is air-sensitive and is reoxidized back to Cu(ll). The resulting set of reactions (Eq. 8.21) are an elegantly simple solution to the problem and resemble the coupled reactions of biochemical catalysis.

Later mechanistic work revealed the following rate equation:
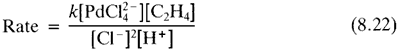
Equation 8.22 implies that the complex, in going from its normal state in solution, PdCl42-, to the transition state of the slow step of the reaction has to gain a C2H4 and lose two Cl- ions and a proton. lt was originally argued that the proton must be lost from a coordinated water, and so that this might undergo olefin insertion into the Pd-OH bond, or the OH might attack the coordinated ethylene as a nucleophile. The resulting hydroxyethyl palladium complex might
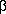 -eliminate to give vinyl alcohol, CH2=CHOH, which is known to tautomerize to acetaldehyde. ln fact, this mechanism is wrong, something that was only discovered 20 years after the discovery of the Wacker process, as a result of stereochemical work by Bäckvall3 and by Stille4. According to the original intramolecular mechanism, whether the reaction goes by insertion or by nucleophilic addition from a coordinated OH, the stereochemistry at each carbon of the ethylene should remain unchanged. This can be tested if we use cis- or trans- CHD=CHD as the alkene and trap the intermediate alkyl. We have to trap the alkyl because the rearrangement to acetaldehyde destroys the stereochemical information. Equation 8.23 shows one way of trapping the alkyl, using CO. You can see that if the hydroxyethyl is carbonylated, the OH group can curl back and effect a nucleophilic abstraction on the acyl to give a free lactone, the stereochemistry of which can be determined by a number of methods, including NMR and microwave spectroscopy. In fact, the stereochemistry of the two carbons in the product is not the same as that of the starting material, which rules out the older mechanisms.
-eliminate to give vinyl alcohol, CH2=CHOH, which is known to tautomerize to acetaldehyde. ln fact, this mechanism is wrong, something that was only discovered 20 years after the discovery of the Wacker process, as a result of stereochemical work by Bäckvall3 and by Stille4. According to the original intramolecular mechanism, whether the reaction goes by insertion or by nucleophilic addition from a coordinated OH, the stereochemistry at each carbon of the ethylene should remain unchanged. This can be tested if we use cis- or trans- CHD=CHD as the alkene and trap the intermediate alkyl. We have to trap the alkyl because the rearrangement to acetaldehyde destroys the stereochemical information. Equation 8.23 shows one way of trapping the alkyl, using CO. You can see that if the hydroxyethyl is carbonylated, the OH group can curl back and effect a nucleophilic abstraction on the acyl to give a free lactone, the stereochemistry of which can be determined by a number of methods, including NMR and microwave spectroscopy. In fact, the stereochemistry of the two carbons in the product is not the same as that of the starting material, which rules out the older mechanisms.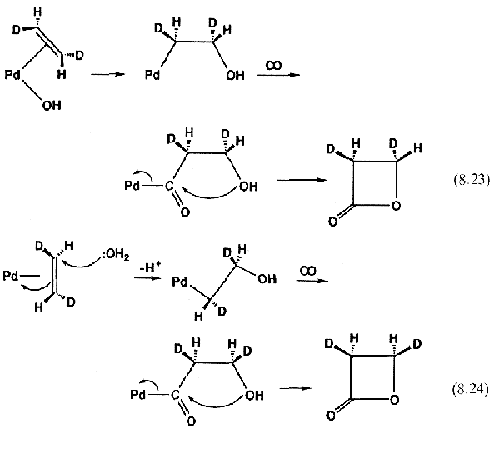
The currently accepted mechanism involves attack of a free water molecule from the solvent on the coordinated ethylene. Equation 8.24 shows how this inverts the stereochemistry at one of the carbons, as opposed to the old insertion mechanism (Eq. 8.23).
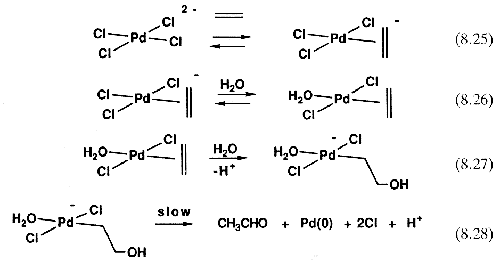
The loss of two Cl- ions removes the anionic charge from the metal, which would otherwise prevent the nucleophilic attack from taking place. Equations 8.25-8.28 show the sequence of events as now understood. This mechanism implies that an [H2O]2 term should be present in the rate equation, and if it could have been seen, the mechanistic problem would have been solved earlier, but one cannot normally alter the concentration of a solvent and get meaningful rate data, because changing the solvent composition leads to unpredictable solvent effects on the rate.
References
[1] F. C. Phillips; Am. Chem. J. 16, 255 (1894)
[2a] J. Smidt; Katalytische Umsetzungen von Olefinen an Platinmetall-Verbindungen; Angew. Chem. 71(5), 176-182 (1959) (../rhodium/pdf /isosafrole-w
[2b] J. Smidt, et. al.; Angew. Chem. 74, 94 (1962)
[3] J. E. Bäckvall, B. Åkermark, S. O. Ljunggren; Stereochemistry and mechanism for the palladium(II)-catalyzed oxidation of ethene in water (the Wacker process); J. Am. Chem. Soc. 101, 2411-2416 (1979) (../rhodium/pdf /wacker.mecha
[4] J. K. Stille, et. al.; Mechanism of the wacker process. Stereochemistry of the hydroxypalladation.; J. Organomet. Chem. 169, 239 (1979) DOI:10.1016/S0022-328X(00)81163-8