05-11-02 02:40
No 307248
Here's a question for you: If styrene/vinylbenzene/phenylethene were subjected to the Ritter Reaction with acetonitrile, what would the product be?
Would it be N-acetyl-phenethylamine? Or would it be N-acetyl-1-methylbenzylamine?
Basically it boils down to which carbon of the ethylene that the nitrogen is going to bond to: the benzyl carbon or the terminal carbon?
I know that for phenylpropenes it bonds to the middle carbon, which corresponds to the terminal carbon of styrene, the desired carbon. But without the third carbon, I can't accurately guess which one it's going to attach to, if the Ritter Reaction is even successful on said compound at all.
It'd be kind of nice to have a way to go from styrene to PEA in one pot, from the ritter reaction, followed by basic hydrolysis of the acetyl group before purification of the (hopefully) formed PEA.
Thanks for your answers.
PrimoPyro
Vivent Longtemps La Ruche!
(Moderator)
05-11-02 13:35
No 307375
Here's a question for you: If styrene/vinylbenzene/phenylethene were subjected to the Ritter Reaction with acetonitrile, what would the product be?
According to Example XXI in US 2,573,673 (Post 262243 (lugh: "Re: Graf-Ritter rxn: allylbenzenes to amph's directly", Novel Discourse))
N-(alpha-phenyl ethyl) acetamide is obtained in 40% yield ![]()
Would it be N-acetyl-phenethylamine? Or would it be N-acetyl-1-methylbenzylamine?
These are two different names for the same compound, thus you would have both ![]()
One mol each of stryene and acetonitrile were dissolved in 200 ml of glacial acetic acid and the resulting solution was added to 500 ml of glacial acetic acid containing in solution one mol of benzene suflonic acid. The resulting solution was allowed to stand several hours or overnight at room temperture. The batch was then added to three or four times its volume of water. The product, N-(alpha-phenyl ethyl) acetamide, was preciptated as a heavy, viscous oil, from which it was removed by distillation in vacuo after neutralization and drying. The distilled product was recrystallized from ligroin and melted at 71-72° C. Yield, 40% of the theoretical amount.
Note that sulfuric acid wasn't used, undoubtedly because of polymerization ![]() Methane sulfonic acid may produce better yields, as may optimzation of the procedure.
Methane sulfonic acid may produce better yields, as may optimzation of the procedure.
(Hive Prodigy)
05-12-02 04:10
No 307586
These are two different names for the same compound, thus you would have both.
Actually, this is not what I implied. One is the structure akin to phenethylamine, the drug, and the other, is a benzylamine.
I see this procedure produces the benzylamine, the undesired compound, sigh. Damn...
Thank you lugh for your answer, it is most informative.
PrimoPyro
Vivent Longtemps La Ruche!
(Chief Bee)
05-12-02 21:54
No 307712
PP: In 99% of alkene addition reactions, Markovnikov's rule is followed, and it has nothing to do with carbon 1, 2 or 3 places away from the end of the chain.
(Hive Bee)
05-13-02 11:03
No 307897
The textbook example of the 1% exception to Markovnikov's rule is the addition of HBr in the presence of peroxides, which brings me to this question:
Is this (anti-M addition of HBr) possible in the case of styrene? Or do other factors make the Br go benzylic anyway?
Hansje high in proteine and fibre!
(Hive Bee)
08-02-02 04:43
No 340036
Dug these up. Seems like as good of a place as any to throw them in.
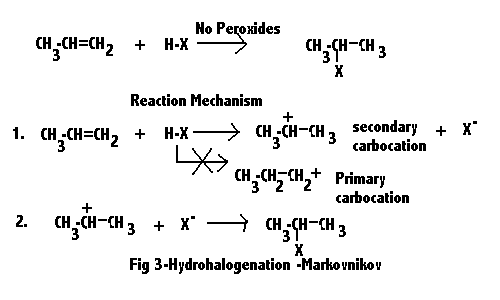
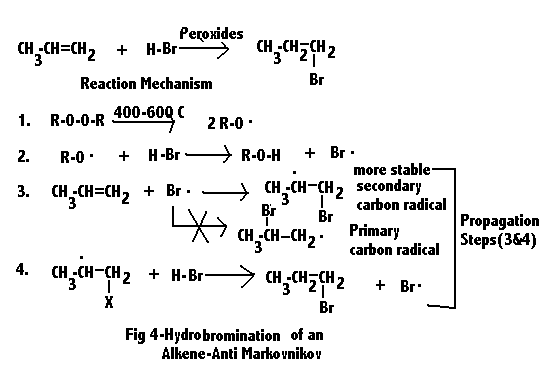
Conclusion /nm./: the place where you got tired of thinking.