09-04-02 21:26
No 352879
ALkenes can be epoxidised by MeCn and H2O2 in MeOH. Can a similar oxygen transporting solvent be used? Ie Dmso??/
Vl_
So much game I could sell a hooker some pussy
Vl_
(Old P2P Cook)
09-04-02 21:55
No 352894
When you use acetonitrile I think that it is being converted to another species which then does the epoxidation.
Edit:
Here we are. The species doing the epoxidation is a peroxyimidic acid formed from the nitrile. See Part 3 - Discussion here:
http://www.orgsyn.org/orgsyn/orgsyn/prep
(Hive Addict)
09-05-02 01:37
No 352944
Does H2O2 and Dmso not form a persulfoxic acid which could do the epoxidation.??
So much game I could sell a hooker some pussy
Vl_
(Hive Bee)
09-05-02 07:45
No 353009
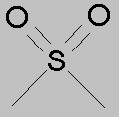
The product of h2o2 oxidation of dimethyl sulfoxide would be dimethyl sulfone. This oxidation would take place in the prescence of a catalyst like sodium tungstate, or phenylphosphonic acid.
Conclusion /nm./: the place where you got tired of thinking.
(Hive Bee)
09-05-02 08:28
No 353020
ABSTRACT:
J.Phys.Chem.A 2000, 104: 3 557 - 561
Detection of Transient Intermediates in the Photochemical Reaction of Hydrogen Peroxide with Dimethyl sulfoxide by Time-Resolved EPR Techniques
Formation of peroxycarboximidic acid (1) is not rate-determining in the reaction of nitrile with alkaline hydrogen peroxide to form amide and oxygen; the yield of amide based on H2O2 varies from 20 to 60%.When dimethyl sulfoxide (DMSO), a reactive substrate, is added, the rate is independent of (DMSO) and governed in turn by a rate-determining addition of HOO- to nitrile.This reaction gives a reliable á-value of kHOO-/kHO-, which is 10000 for benzonitrile.A facile conversion of nitrile to amide may be achieved by the reaction in the presence of DMSO, unacco mpanied by side reactions such as the epoxyamide formation from á,â-unsaturated nitrile.Kinetics and product analysis suggest that a predominant reaction is not a non-radical oxidation of H2O2 with 1 but a radical decomposition of H2O2 which is induced by the homolysis of anion of 1 (1A).No singlet oxygen could be trapped chemically.The reaction of superoxide ion, O2-., with acetonitrile is shown to be analogous to that of HOO-; the decomposition of O2-. is fast in the presence of MeCN and DMSO in benzene, affording acetamide and dimethyl sulfone.
I'm thinking that you could take a nitrile, and using the above synthesis convert this to an amide in high yeilds, and follow that with a reduction or Hofmann rearrangement to yeild an amine.
It's an idea anyway. I guess i'm just waiting for someone to shoot me down or tell me that someone has been there and done that and got a t-shirt.
Flip
Conclusion /nm./: the place where you got tired of thinking.
(Hive Addict)
09-06-02 10:43
No 353538
Swim would try it any way. The UV will open the double bond to attack. And the H2O2 Will be there to attack.
So much game I could sell a hooker some pussy
Vl_
(Stoni's sexual toy)
09-17-02 07:49
No 357304
If you guys want to oxidise alkenes it might be a good idea NOT to use DMSO as the solvent, especially since alcohols work very well and are much cheaper too.
UTFSE, this reaction has been done by at least one bee (sunlight?) in the past, there should be more information about it on these boards.
I'm not fat just horizontally disproportionate.