10-04-02 04:35
No 364034
In a recent message in alt.drugs.ghb the idea of turning MSG to GHB was discussed. Here is the reference:
http://groups.google.ca/groups?q=msg+ghb
The above reference refers to a message on Ceri, here:
http://www.ceri.com/alabama3.htm
Which states:
monosodium glutamate (MSG), the essential amino acid, dietary supplement and flavor enhancer which converts to GHB in one step using nitrous acid (HNO2, a deaminating agent)
Ok, so turning NH2-CH(CH2CH2COOH)-COO- Na+ into HOCH2CH2CH2COO- Na+ seems kinda far-fetched to me. Sure nitrous acid in the presence of sulfuric acid can nitrosate alkylamines, but you don't hear of many of these reactions since the N-nitroso compounds decompose to the diazonium salt... which is unstable and break down to yield a carbocation.
Would it be possible that the carbocation would react with water, eliminate the alpha-C (in the form of CO2) and form GHB?
If not, is it possible that this reaction would work if MSG were decarboxylated to GABA--can anyone suggest a way other than GAD (glutamate decarboxylase)? Then react GABA with nitrous acid? NaGlu is cheap and everywhere, it would be cool if this rxn worked.
... even if there was an easy way to get GABA from MSG it would be of interest to me.
(Chief Bee)
10-04-02 05:50
No 364051
Would it be possible that the carbocation would react with water, eliminate the alpha-C (in the form of CO2) and form GHB?
That could happen, that could happen.
(Synaptic Self-Mutilator)
10-04-02 22:36
No 364286
It looks like adding nitrous acid to NH2-CH(R)-COOH will make HO-CH(R)-COOH as in:
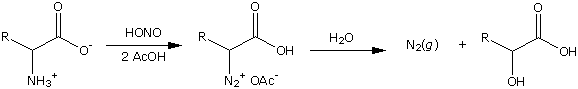
If the diagram is right, it appears that the result would be 2-hydroxyglutarate not gamma-hydroxybutryate. (ie HO-CH(CH2-CH2-COOH)-COOH not HO-CH2-CH2-CH2-COOH). It appears that reacting GABA with nitrous acid would make a mixture of crap (including GHB) as in:
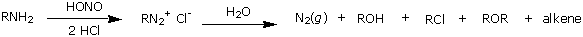
Now, ok, 2-hydroxyglurate can be made... but is that good for anything? Not really, my bets are on it entering the Krebb's cycle and being turned into CO2 & H2O.
It also came to my attention when searching that heating monosodium glutamate will produce sodium pyroglutamate. (sodium salt of pyroglutamic acid). Heating GABA will also produce a lactam (cyclic amide). Damn, damn & damn.
I'd love for someone to tell me that I'm wrong!
(Chief Bee)
10-04-02 22:59
No 364288
Reactant: 4-amino-butyric acid
Products: dihydro-furan-2-one (70%), 4-hydroxy-butyric acid (30%)
Reagents: NaNO2, HCl
Solvent: H2O
Time: 24 hour(s)
Other Conditions: Ambient temperature
Reference: Bull.Soc.Chim.Fr.; 1; 88-94 (1989)
Reactant: butyric acid
Product: 4-hydroxy-butyric acid, dihydro-furan-2-one
Reagent: O2, K2PtCl4, K2PtCl6
Time: 144 hour(s)
Temperature: 80-90°C
Other Conditions: other aliphatic carboxylic acids; also without Pt(IV)-catalyst
Reference: J.Chem.Soc.Chem.Commun.; 18; 1991; 1242-1243.
Reactant: succinic acid monomethyl ester
Product: 4-hydroxy-butyric acid
Yield: 90 percent
Reagent NaBH4
Solvent: H2O
Time: 12 hour(s)
Other Conditions: Ambient temperature
Reference: Synth.Commun.; 18; 15; 1988; 1765-1772.
Reactant: succinic acid anhydride
Product: 4-hydroxy-butyric acid
Reagent: hydrogen, nickel
Other Conditions: die Saeure bezw.ihr Lacton, das Butyrolacton entsteht
Ref: Chem.Zentralbl.; GE; 78; I; 1907; 1617.
Reactant: succinic acid anhydride
Product: 4-hydroxy-butyric acid
Reagent: sodium amalgam, diethyl ether, hydrochloric acid
Other Conditions: die Saeure bezw.ihr Lacton, das Butyrolacton entsteht
Reference: Chem.Ber.; 29; 1896; 1193.
(Synaptic Self-Mutilator)
10-04-02 23:47
No 364302
It's good to be wrong! GBL and GHB in 100% yields from NaNO2/HCl and GABA, excellent!
Now please, someone show me how to turn Na-Glu into Na-GABA.
But, luckily even GABA is available in 100ct bottles of 750mg capsules for moderately reasonable prices. I should check to see if the local nutrition store carries it...
If so, then it's definitely time to polish up on my French and check out the Bulletin de la Société chimique de France to find out the details on the rxn.
I was definitely discouraged to check out the refs from Oxidations in Organic Chemistry by Hudlicky on the oxidation of cyclic ethers, because the references for CrO3 and KMnO4 did not apply to cyclic ethers (which I find really odd!). This has brought my hopes up again!
Thanks Rhodium.
(Synaptic Self-Mutilator)
10-05-02 01:04
No 364323
My nutrition store came up short! Where is the GABA?
I found out that's an awesome reference. I confirmed the 30% GHB & 70% GBL from GABA (80% and 20% from GABA-OMe), and read that you get 100% of 2-hydroxyglutarate lactone from Glu.
The experimental reads:
Des solutions aqueuses equimoleculaires en nitrite de sodium et acide amine sont realisees a une concentration de 0,5 M ou 1 M suivant la solubilite des acides amines. Le pH de la solution est ajuste a la valeur desiree (pH = 4-5) avec HCl 2N. Le melange est agite a temperature ambiante pendant 24 heures afin que le degagement gazeux soit termine. Puis la solution resultante est analysee par RMN 13C.
Which I think roughly means:
Aqueous equimolar solutions of sodium nitrite and amino acid are made up at 0.5 M or 1 M depending on the solubility of the amino acid. The solution is adjusted to pH = 4-5 with 2N HCl. The mixture is stirred at room temperature for 24 hours (until the nitrogen emission is done). Then the solution is analyzed by carbon-13 NMR.
How do I remove that carboxy group? Anyone? Please!
(Synaptic Self-Mutilator)
10-05-02 01:27
No 364327
Suggested experimental:
103.1g GABA and 69.0g NaNO2 is added to 1L of water. Stirring is started. 116.7g of 31.25% HCl is slowly added. pH is checked to make sure it's around 4-5 (if not add more HCl). Reaction is run 24hrs with no heating.
The expected yield is 86.1g of GBL. The whole mixture can be distilled to dryness, then 84.0g of NaHCO3 is added to the distillate, refluxed for 30 minutes and boiled down to a reasonable volume and enjoyed responsibly.
In the case of MSG, 2 mol of 31.25% HCl is added (233.3g) and 1 mol of MSG (169.1g). This unfortunately won't make GHB.
Now, who's gonna try this out?!?
(Hive Bee)
10-05-02 04:54
No 364402
So GAMA is just gamma amino butyric acid, or 4 amino butyric acid? And it can be found at the local drug store? is there a special name they call it? PM me, Chromic, immediately.
Who is that masked man?
(Synaptic Self-Mutilator)
10-05-02 05:00
No 364404
This reaction appears to be quite exothermic. I'm doing it on a 0.1 mole scale with MSG. I'd highly recommend anyone attempting this to slowly drop in the hydrochloric acid. I can see bubbles of what I hope are N2. Very nice, very easy!
EDIT: external cooling is definitely necessary! Sit your flask in some cold water otherwise you'll be breathing in NO2!
(Hive Bee)
10-06-02 18:44
No 365071
I wonder if a monomethylamine can be utilized in such a reaction, if it is the case we can use the open form of 1-Methyl-2-pyrrolidinone (NMP) for the replacement of ol' GBL
(Hive Addict)
10-07-02 00:03
No 365170
Zoro: GABA = gamma amino butyric acid. It is available at every health store and supermarket that I have encountered in my area.
This is a very exciting thread!! Thanks Chromic, Rodium and everyone else who has participated!!
The above post is purely fictional. Any resemblance to "real-life" is purely coincidental.
(Synaptic Self-Mutilator)
10-07-02 05:37
No 365279
I think you mean pyrrolidone as a precursor for GHB (ie GABA lactam)--not NMP.
I gotta find me some GABA. This reaction is a breeze, the test with MSG went splendidly!
10-08-02 01:38
(Rated as: insignificant)
(Hive Bee)
10-08-02 03:54
No 365755
Does GBL steam distill? Because if it does, then distillation should be a breeze, but you'll be left with some impure GBL. If not, then it should be easy to distill it to reasonable purity, since it's BP is so much higher than anything else in the reaction mix.
Who is that masked man?
(Synaptic Self-Mutilator)
10-08-02 04:51
No 365782
>Does GBL steam distill?
Yes, 1 part GBL and 9 parts water seems to do it alright... which is EXACTLY what's called for in the reaction. Distill off the water/GBL and react the distillate with a base (eg sodium bicarbonate).
It's a very efficient way of purifying everything...
(Hive Bee)
10-08-02 18:39
No 366079
Poix: A secondary nitrogen like the one in decyclised NMP, won't diazotise, so it won't be of any help.
(Synaptic Self-Mutilator)
10-21-02 21:27
No 370961
I wish decarboxylation could only be as simple as adding a dash of MSG to flavor up some immobilized Escherichia coli to produce the desired gamma-aminobutyric acid... (ie that you didn't need an ion exchange resin to work up the product)
So, I gather we're all pretty sure that there's no easy way to decarboxylate MSG?
(Stranger)
10-23-02 21:54
No 371854
Des solutions aqueuses equimoleculaires en nitrite de sodium et acide amine sont realisees a une concentration de 0,5 M ou 1 M suivant la solubilite des acides amines. Le pH de la solution est ajuste a la valeur desiree (pH = 4-5) avec HCl 2N. Le melange est agite a temperature ambiante pendant 24 heures afin que le degagement gazeux soit termine. Puis la solution resultante est analysee par RMN 13C.
This is what I get, from http://www.freetranslation.com :
Aqueous solutions equimoleculaires in sodium nitrite and acid amine are realisees has a concentration of 0,5 M or 1 following M the solubilite of the acids amines. The pH of the solution is adjusts has the value desiree (pH = 4-5) with HCl 2N. The mix is shakes has ambient temperature during 24 hours so that the gaseous degagement be finishes. Then the solution resultante is analysee by RMN 13C.
TTT
(Synaptic Self-Mutilator)
10-23-02 22:16
No 371869
Thethethe, I think what I wrote:
Aqueous equimolar solutions of sodium nitrite and amino acid are made up at 0.5 M or 1 M depending on the solubility of the amino acid. The solution is adjusted to pH = 4-5 with 2N HCl. The mixture is stirred at room temperature for 24 hours (until the nitrogen emission is done). Then the solution is analyzed by carbon-13 NMR.
Should make a lot more sense to anyone who speaks English.
(Stranger)
10-24-02 03:41
No 372001
I think that translation is more akin to a dyslexic, ebonic-speaking, french-ghetto, monk-chemist; still, perhaps the freetranslation site can help some get a basic understanding of some foreign synths...
TTT
(Hive Bee)
10-26-02 23:17
No 372974
Rhodium posted this refernce:
Reactant: 4-amino-butyric acid
Products: dihydro-furan-2-one (70%), 4-hydroxy-butyric acid (30%)
Reagents: NaNO2, HCl
Solvent: H2O
Time: 24 hour(s)
Other Conditions: Ambient temperature
Reference: Bull.Soc.Chim.Fr.; 1; 88-94 (1989)
I was wondering is the major product 'dihydro-furan-2-one'
GBL under a different name?
Or am I mistaken.
(Chief Bee)
10-26-02 23:39
No 372981
Yes, that's a GBL synonym.
(Hive Bee)
10-27-02 00:19
No 372996
That's really cool then, so the nitrous acid forms a diazonium salt which then gets hydrolysed to N2 and H2O, and hydroxy-butyric acid.
I not sure I understand the mechanism for this, but that's some neat stuff for sure!
(Hive Addict)
10-27-02 23:32
No 373344
great way to store your solid ghb... freeze first then vacuum pack it... nice way to handle.. no h20 pickup
Infinite Radiant Light - THKRA
(Hive Bee)
11-02-02 21:08
No 375794
SWIM would very much like to try this rxn. As far as the purification of the formed GBL is concerned, when steam distillation is performed will the 30% formed GHB distill over with the GBL? SWIM would assume/hope so, but just wanted to make sure. Obviously the Na-GHB salt won't steam distill but the free compound formed in the reaction will, right? Thanks
(Chief Bee)
11-02-02 21:13
No 375796
If you acidify the solution slightly (pH 4-6), then all of the GHB will turn into GBL eventually in the distillation and steam distill.
(Hive Elder)
11-03-02 19:12
No 376081
Below is a quote from the following page
http://www.msgtruth.org/body.htm
Pancreas – glutamate stimulates the pancreas and may cause Type II diabetes, obesity, and insulin resistance. In individuals with Type 1 diabetes, it may cause an excess of glutamate since 85% of those with Type 1 diabetes have antibodies which attack GAD - the enzyme responsible for converting glutamate into GABA.
How hard would it be to get some of that enzyme and set it to work converting your MSG into GABA...? MSG is dirt cheap in comparison to purchasing GABA unless the process for conversion takes too much effort... Any thoughts...?
(Synaptic Self-Mutilator)
11-03-02 22:43
No 376126
>I wish decarboxylation could only be as simple as adding a dash of MSG to
>flavor up some immobilized Escherichia coli to produce the desired gamma-aminobutyric
>acid... (ie that you didn't need an ion exchange resin to work up the product)
Sorry, it seems as thought my comments were spot on. The enzyme you speak of is in E. coli btw.
(Hive Elder)
11-04-02 00:46
No 376159
So it would be a hassle to go that route then...? What would workup involve...?
(Synaptic Self-Mutilator)
11-04-02 05:03
No 376228
Amino acids are hard to purify as they have both an amino group and an carboxylic acid group. They aren't easily purified by acid/base base techniques. One common method, and one used in the reference I found the information of GAD existing in E. coli mentioned the use of an ion-exchange resin. I suppose if you had isolated GAD you would not need to purify it further. Unfortunately the purified enzyme would likely cost a fortune.
(Stranger)
07-06-03 21:59
No 444956
If the NxOy gases produced were bubbled back through the GABA + H2O + Na+ + H3O+ + CL- + NO3- reaction solution in a closed system...
Fit at the mouth of a 1 liter flask a pressure-driven, accordian style bladder/gas balloon. When the volume of gas increases pressure, a pressure valve opens, the bladder deflates, and the volume of gas is directed via a glass tube down into and bubbled through the solution.
The piped gases would stir the reaction solution and perhaps replenish the reaction with H3O+ via H2O(g) returning and ionizing with dissolved NxOy gasses.
External cooling would be even more important with this setup.
Wouldn't this make the reaction more "closet," or at least "bathroom," friendly? Does such a piece of equip. exist? Would it help speed up the reaction or require less NaNO2?
Or, will it hinder GBL production?