11-04-02 23:57
No 376470
Anyone have access to "Green Chemistry" published by the Royal Society of Chemistry? This abstract looks pretty neat...now maybe green methylations will bee possible for bees?
Green Chemistry 2002 4(5) p.431-5
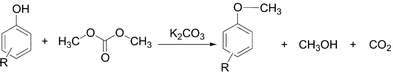
Dimethyl carbonate and phenols to alkyl aryl ethers via clean synthesis
Samedy Ouk , Sophie Thiébaud , Elisabeth Borredon and Pierre Le Gars
The industrially important alkyl aryl ethers (ArOR) were selectively obtained in good yields from the O-alkylation of the corresponding phenols with the environmentally benign reagents, dimethyl carbonate or diethyl carbonate. The reactions were carried out under atmospheric pressure, in a homogenous process, without solvent and in the presence of potassium carbonate as catalyst.
(Chief Bee)
11-05-02 00:11
No 376474
../rhodium/pdf /dimethylcarbo
(Hive Bee)
11-05-02 15:23
No 376697
I'm sure that many bees who would like to synth 2,5 DMBA do not want to use DMS or MeI due to their toxicity and carcinogenic effects.
how well would this work for methylating 2-hydroxy 5-methoxy benzaldehyde to form 2,5 DMBA?
Could the process be adapted to work in a simpler apparatus, or is a variable take-off still head necessary in this case?
Am I right in thinking that if adaptable, this process would be better (less toxic) thank trimethyl phosphate methylations?
Which would you prefer to work with? (DMC is less toxic but far more volatile boiling at 90 dec C as opposed to 197 deg C).
(Chief Bee)
11-05-02 18:21
No 376738
Personally, I have no problem whatsoever working with Me2SO4 - it is not especially volatile at room temp, and if you are wearing rubber gloves while handling it, you won't ingest/inhale/absorb any of it, and it's dirt cheap - about $20/kg. This assumes that you can buy dimethylsulfate. I would not reccommend synthesizing it yourself in the kitchen.

(Hive Bee)
11-05-02 18:48
No 376747
well I for one can't buy DMS easily
and I don't think working with MeI is good without a fume hood (volatile).
DMS also poses a waste disposal problem to me.
DMC is also very cheap at around $18/kilo
TMP is not that expensive at around $42/kilo
I would always prefer to use less toxic chemicals if there is not a major disadvantage.
so if you or someone else are able to help with the questions I raise above I believe that it would be useful to me and others.
(Chief Bee)
11-05-02 18:52
No 376750
What kind of waste disposal problem does DMS pose? Just reflux in some NaOH and you get methanol and sodium sulfate...
(Hive Bee)
11-05-02 19:05
No 376755
I didn't know that I could do this.
I would still prefer to use something that I can dispose of more easily rather than first having to handle several hundred milliliters of highly toxic reagent.
Anyway, I suppose its academic how I dispose of it if I can't even buy it easily.
(Hive Adickt)
11-05-02 20:00
No 376775
Any methylation regent is toxic. if it's capable to methylate phenol it's also capable to methylate your DNA.
DMS is wonderfull to work with, cheap and reactive(easy to destruct) MeI is harder to destruct and therby more caracinogenic.
(Chief Bee)
11-05-02 20:12
No 376778
MeI is also more expensive, and not as stable on prolonged storage.
Does anyone have any data on the relative electrophilicity of MeI, Me2SO4, Me3PO3 & Me2CO3?
(Hive Bee)
11-05-02 21:12
No 376799
Hest: I understand that any methylation agent will be toxic, but some are more toxic than others.
Looking at available MSDS/toxicity data I can find and taking into account the opinions of researchers, it appears that DMC is considerably less toxic than DMS. However DMC is significantly more volatile. It appears that TMP is also less toxic than DMS (but more toxic than DMC).
As to nucleophilicity, I'm not sure but will see what I can dig up when I go to the library.