01-06-03 05:19
No 395791
I've decided to put the pedal to the metal and synth. this MF. I've decided to react Grignard complex with (S)-proline ethyl ester:
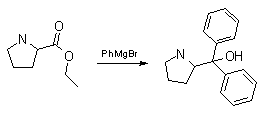
However, I have only proline so I'll have to synth the Et-ester of it mytself.
I have no refs on the esterification part of this particular amino acid but I intend to apply what I know in general. This what I have written in my lab-record-book and what I'm about to follow:
Procedure:
Nearly equimolar amounts of (S)-proline and EtOH are placed in a 500 mL RB flask (slight excess of EtOH, I want proline to be limiting since EtOH is wiledly available :). Concentrated H2SO4 is added to the reaction mixture. Mixture is heated under re-flux for about 3 h (to be certain, I have no TLC plates at the time, thus I can not develop TLC).
When 3 h has passed the reaction flask is cooled and the reaction mixture is poured over crushed ice (in a baker). This is stirred around with a glas rod for about a minute or two and transfered to a 200 mL sep. funnel. To minimize losses and to get better yield both reaction flask and ice-baker are rinsed with ether and the washing poured into the sep. funnel. Aqueouse phase run off the drain, and the org. phase is washed with freshly prepared FeSO4 solution and then with 2 times with sodium carbonate solution. Ether phase is dried over MgSO4, filtered and the solvent is striped off under vacuuo. For the sake of quality and pride the residue (crude product) should be distilled once more.
What do you think about the above?

I'm more interested in what you think about my new DEA-HAT!
Accept No Imitations, There Can Only Be One; www.the-hive.ws
(Newbee)
01-06-03 06:34
No 395803
Way to go pHarmacist
I was hoping some bee would try this project ... I am like very interested in a bioassay of this compound.
I once prepared the methyl ester of phenylalanine by bubbling HCl (g) in a methanol solution of Phe. If you 're interested, I 'll fetch the procedure from my notes.
Nearly equimolar amounts of (S)-proline and EtOH are placed in a 500 mL RB flask (slight excess of EtOH, I want proline to be limiting since EtOH is wiledly available :).
Why use equimolar amounts? You need a large excess of alcohol to drive the equilibrium of the Fischer esterification to the right. Use the ethanol as solvent.
Diazomethane can be used to prepare methyl esthers in rather quantitative yield ... unfortunately it's such a violent poison
Keep us informed
(Hive Addict)
01-06-03 07:57
No 395816
A nice procedure would be great!
(Hive Bee)
01-06-03 10:03
No 395847
The target compound is commercially available. I've bought a gram last year and evaluated it together with SWIM. Yes, it is stimulating and pretty strong (5-10 mg lasts several hours), but completely different than the amphetamine type of stimulation. There is no need to repeat this experience, no pleasant kick or euphoric rush. I'm very happy that I was able to get this knowledge before I've started to waste a lot of $ and time. There are better things to do with chemicals and time, IMHO.
(Chief Bee)
01-06-03 10:27
No 395852
We can still reduce the alcohol to the hydrocarbon to get the benzhydryl-pyrrolidine. It might still be good.
Kick/Euphoria aside, could it be used as a study aid?
(Hive Bee)
01-07-03 01:00
No 396075
IIRC, the reduced compound is much weaker (abt. 10 times). Considering the high price (abt. 30$/gram) of the intermediate, the reduction of this strong (but not too pleasant) compound would be a waste of money.
(Hive Addict)
01-07-03 03:07
No 396100
Hi all!
Thanks to lugh for fetching and Rhodium for sending me the references I requested in Novel Discourse. However, I got dissapointed when Nemo_Tenetur provided us with the bioassay.
Preparation of working Grignard was futile. I have never failed preparing a Grignard before, but this time I'm certain that Mg-tunings were bad. I made a friend order new, but I don't think that I'll continue on this project since the product is - according to Nemo's experience - waste of money/time. Thank you all for your support.
Case closed.
EDIT: Rhodium, I remember you mentioning some other cocaine mimmic. You said that the article (published in JACS/JOC ?) describing the synthesis become mysteriously unavailable. Foxy2 promised (
Accept No Imitations, There Can Only Be One; www.the-hive.ws
(Chief Bee)
01-07-03 03:28
No 396106
Post 329215 (Rhodium: "American Chemical Society censors drug synths!", General Discourse)
Post 339090 (Rhodium: "ACS Censors drug synths - Part II", General Discourse)