(Hive Bee)
05-16-03 19:55
No 433581
(Rated as: excellent)
This is a requested ref, Post 433308 (not existing)
Journal of Organometallic Chemistry 323 (1987) 145-147
A Simple Convenient Method for the Generation of Diboranre from NaBH4 and I2
C. Narayana and M. Periasamy
Summary
Treatment of NaBH4 with I2 in diglyme yields diborane which can be utilized conveniently for the perparation of a borane-N,N-diethylaniline complex and other borane-Lewis base complexes.
In the course of our studies on the hydroboration reaction [1,2], we decided to seek a convient method for the synthesis of borane-Lewis complexes. Freeguard and Long reported in 1965 that iodine reacts with NaBH4 in diglyme at room temperature to give diborane [3] (eq. 1).
diglyme
I2 + 2NaBH4 ---------> B2H6 + 2NaI + H2 (1)
r.t.
The authors utilized a vacuum-line technique and isolated the diborane using a series of liquid nitrogen traps [3]. They reported that the yield of diborane was > 90% and that the product was not contaminated with any detectable impurities [3]. They suggested that this method would be more suitable for the preparation of pure diborane than the commonly employed method involving BF3OEt2 and NaBH4 since the product in the latter case contains small amounts of BF3 and Et2O. In addition, the I2/NaBH4 system has the advantage that I2 is easier to handle than BF3OEt2. In spite of its attractions there seems to be no report of the utilization of this method in organic synthesis. This may, as suggested by Lane [4] be because of the lack of a detailed account of the experimental procedure for the use of the I2/NaBH4 system. In addition, the use of a vacuum-line technique by the original authors [3] may have given the impression that the I2/NaBH4 system might not be suitable for generation of diborane for use in bench-top organic synthesis.
We wish to report that diborane can be readily generated using the I2/NaBH4 system in an apparatus similar to that used for the BF3OEt2/NaBH4 system [5,6]. The generated diborane can be readily utilized for the preparation of N,N-diethylaniline complex in benzene or toluene (see Experimental). We have encountered no difficulty in the preparation of BH3-THF on a 100 mmol scale for utilization in the reduction of tertiary amides and imines.
TABLE 1: Hydroboration-Oxidation of alkenesa
| Substrate | Product | Yield % b |
| n-C8H17CH=CH2 | n-C8H17CH2CH2OH c | 82 |
| n-C4H9CH=CH2 | n-C4H9CH2CH2OH | 78 |
| C6H5CH=CH2 | C6H5CH2CH2OH d | 72 |
| Cyclohexene | Cyclohexanol | 72 |
Bicyclo[2.2.1]hept-2-ene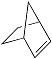 |
Bicyclo[2.2.1]heptan-2-ol 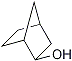 |
67 |
2,6,6-trimethyl-bicyclo[3.1.1]hept-2-ene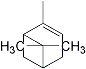 |
2,6,6-trimethyl-bicyclo[3.1.1]heptan-3-o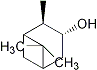 |
68 |
| CH2=CH(CH2)8COOCH3 | HOCH2CH2(CH2)8COOCH3e | 80 |
a For all substrates, 10 mmol od N,N-diethylaniline-borane complex was used in toluene (or bezene) (30 ml). Hydroborations were carried out with 30 mmol of terminal alkenes and 20 mmol of internal alkenes at room temperature for 3 h then at 50° C for 1 h to ensure complete hydroboration. Oxidations were carried out using 30% hydrogen peroxide/NaOH after the addition of methanol (2 ml) and THF (20 ml) following the reported procedure [6].
b Yields are of the products isolated after distilation.
c The crude product obtained on oxidation with diethyl ether/chromic acid system [7] gave 2-decanone in 4% yield.
d Product contains 20% 1-phenylethanol (1H NMR).
e Oxidation was carried out using NaOAc/H2O2.
The hydroboration-oxidation of representative alkenes using the N,N-diethylanilineborane complex, prepared as above , gives the corresponding alcohols in good yields (Table 1). The amineborane complexes are relatively stable, and usually bring about hydroboration of alkenes at elevated temperatures (> 100° C) [8,9]. However, it has been known for some time that a few complexes such as N,N-diethylaniline- and N-phenylmorpholine-borane bring about hydroboration at room temperature [9]. The method decribed here of preparing borane-Lewis base complexes in hydrocarbon solvents should be attractive to practising chemists [10]. In addition, the method also provides a convenient source of diborane gas, free from contaiminants such as BF3 or Et2O, and hence should be more suitable for applications in organometallic chemistry.
Experimental
The benzene, toluene, and diglyme were distilled over benzophenone sodium immediately before use. The N,N-dithylaniline was purified by distillation over KOH. Sodium borohydride, iodine, and olefins were commercial samples.
Procedure for diborane generation
A solution of iodine (10 mmol) in diglyme (10 ml) was introduced dropwise during 20 min into the generator flask containing NaBH4 (20 mmol) in diglyme (10 ml) at room temperature (tap waster cooling) under a static nitrogen atmosphere.
The generated diborane and hydrogen were carried off through a side tube and bubbled through a solution of N,N-diethylaniline (10 mmol) in toluene or benzene (30 ml) in another flask at 0° C. The outlet from the latter flask was vented through a mercury bubbler and a trap containing [an] adequate amount of acetone to destroy excess diborane. When the bubbling of the gasses in the reaction flask had ceased, the bubbler was removed under nitrogen and replaced by a glass stopper. the bubbler was connected to an acetone trap and excess diborane in the generator flask was driven off with a stream of nitrogen. The diborane in the gas phase above the toluene (or benzene) solution in the reaction flask ws flushed out with a stream of nitrogen. The N,N-diethylaniline-borane complex prepared in this way when treated with Ph3P gave the Ph3PBH3 complex in essentially quantitative yield.
References
1. C. Narayana and M. Periasamy, Tetrahedron Lett., 26 (1985) 1757.
2. C. Narayana and M. Periasamy, Tetrahedron Lett., 26 (1985) 6361.
3. G.F. Freeguard and L.H. Long, Chem. Ind., (1965) 471.
4. C.F. Lane, Chem. Rev., 76 (1976) 773.
5. H.C. Brown and R.L. Sharp, J. Am. Chem. Chem. Soc., 90 (1968) 2915.
6. H.C. Brown, Organic Synthesis via Boranes, Wiley-Interscience, New York, 1975.
7. H.C. Brown, C.P. Garg and K-T. Liu, J. Org. Chem., 36 (1971) 387.
8. A. Pelter and K. Smith in, D.H.R. Barton and W.D. Ollis (Eds.), Comprehensive Organic Chemistry, Pergamon Press, Oxford, 1979.
9. Unpublished observations by C.F. Lane as mentioned in ref. 8, vol 3, p 729.
10. M. Follet, Chem. Ind., (1986) 123. This article summarizes the problems involved in the generation and handling of diborane and borane-Lewis base complexes.
Got democracy?http://www.dhushara.com/book/multinet/de
(Hive Addict)
05-19-03 13:18
No 434135
Borane can be generated in situ from NaBH4 and almost any lewis acid that is not reactive with borane.