07-09-03 23:48
No 445951
PMHS (poly(methylhydrosiloxane), polymethylhydrosiloxane, polymethylhydrogensiloxane) has been mentioned at the Hive few times, see
Post 170334 (Lilienthal: "Novel reductive amination with PMHS-Ti(OiPr)4", Novel Discourse)
Post 184126 (smiley_boy: "Re: Dimethyltryptamine", Tryptamine Chemistry)
Inspired by the the review "Polymethylhydrosiloxane: a versatile reducing agent for organic synthesis"
(J. Chem. Soc., Perkin Trans. 1, 1999, (23), 3381 - 3391 (http://www.angelfire.lycos.com/scifi2/l
by Nicholas Lawrence et al. some synthetic methods which might bee of interest here were selected.
PMHS has several advantages over other reducing agents. Metal hydrides (LAH, NaBH4, Red-Al) are flammable, difficult to handle (LAH), toxic (NaBH4) and often watched. Reductions with H2 and a metal catalyst often require high pressure, or the catalyst itself is pyrophoric (Raney-Nickel) or expensive (Pd-salts). Other reducing agents used in clandestine chemistry (Al/Hg, Zn, SnCl2, hydrazine, ...) can often only bee used for certain reactions.
It is this lack of reactivity in the absence of catalysts that makes PMHS such an attractive reducing agent (Lawrence, 1991)
PMHS is a colourless liquid with a density of 1 g/ml with a flash point > 100 °C. It is non-toxic and stable to air and moisture.
PMHS is not too expensive, about 50$ for 1 litre.
Some selected reactions
A reductive amination with Ti(i-PrO)4 has already been mentioned by Post 170334 (Lilienthal: "Novel reductive amination with PMHS-Ti(OiPr)4", Novel Discourse)
Another reductive amination with ZnCl2 and PMHS is described in Synthetic Communications, 1999, 29(22), 3981-3987 (http://www.angelfire.lycos.com/scifi2/l
Table 1
| Entry | Substrate | Product | Yield (%)a | imine (mg), (mmol) | Reaction time (h) |
| 1 | Molecule: A1a ("c1c(cccc1)/N=C/c2ccccc2") |
Molecule: B1a ("c1c(cccc1)NCc2ccccc2") |
67 | 181, 1 | 10 |
| 2 | Molecule: A2a ("c1c(ccc(c1)OC)/N=C/c2ccccc2") |
Molecule: B2a ("c1c(ccc(c1)OC)NCc2ccccc2") |
71 | 195, 1 | 12 |
| 3 | Molecule: A3a ("c1(ccccc1)C/N=C/c2ccccc2") |
Molecule: B3a ("c1(ccccc1)CNCc2ccccc2") |
73 | 211, 1 | 10 |
| 4 | Molecule: A4a ("c1(ccccc1)/N=C/C=C/c2ccccc2") |
Molecule: B4a ("c1(ccccc1)NC/C=C/c2ccccc2[mol=B4a]") |
55 | 207, 1 | 20 |
| 5 | Molecule: A5a ("c1(ccccc1)[C@H](/N=C/C=C/c2ccccc2)C ") |
Molecule: B5a ("c1(ccccc1)[C@H](NC/C=C/c2ccccc2)C") |
50 | 235, 1 | 24 |
| 6 | Molecule: A6a ("c1(ccccc1)/N=C(/C)c2ccccc2") |
Molecule: B6a ("c1(ccccc1)NC(C)c2ccccc2") |
60 | 195, 1 | 15 |
| 7 | Molecule: A7a ("c21ccccc1c(ccc2)/C=N/Cc3ccccc3") |
Molecule: B7a ("c21ccccc1c(ccc2)CNCc3ccccc3") |
63 | 245, 1 | 12 |
| 8 | Molecule: A8a ("c1c(ccc(c1)OC)\N=C/c2c(cccc2)Cl") |
Molecule: B8a ("c1c(ccc(c1)OC)NCc2c(cccc2)Cl") |
70 | 245, 1 | 12 |
| 9 | Molecule: A9a ("c1c(cccc1)/N=C/c2c(cccc2)[N+]([O-])=O" |
Molecule: B9a ("c1c(cccc1)NCc2c(cccc2)[N+]([O-])=O") |
75 | 225, 1 | 12 |
a yields are based on isolated chromatographically homogeneous products
General
All reactions were conducted under an inert atmosphere.
General procedure for the preparation of imines
The carbonyl compound (1.0 equiv.) and amine (1.1 equiv.) were dissolved in dry toluene under nitrogen in a round-bottom flask equipped with a reflux condenser and a Dean-Stark trap. The mixture was heated to reflux for 24-28 hours. The solvent was then removed under reduced pressure and the imine products obtained were used without further purification.
Preparation of imines (Entry 4,5 and 6)
To about 1 g of activated molecular sieves (4 Angstrom) in 50 ml round bottom flask were added 15 ml of benzene, a carbonyl compound (2 mmol) and an amine (2.2 mmol) and the mixture was stirred at ambient temperature for 20 h. The molecular sieves were filtered off, the volatiles were removed on a rotary evaporator to yield the imine in quantitative yield. The imines thus obtained were utilized for further reductions without further purification.
General procedure for the reduction of imines
To polymethylhydrosiloxane (PMHS) (300 mg) in a 25 ml flask fitted with septum inlet and magentic stirr bar, was added freshly fused ZnCl2 (270 mg, 2 mmol) in 5 ml dry ether under nitrogen atmosphere. After 10 min the imine (1 mmol) was added and the reduction was allowed to stir at room temperature as specified. The reaction mixture was extracted with 1M HCl (2x 15 ml). The aqeuous layer was further washed with CH2Cl2 (15 ml) to remove non-amine impurities. The purified aqeuous layer was basified to pH ~10 with 1N NaOH, and extracted with ethyl acetate (3x 15 ml). The combined organic layers were washed with water (1x 15 ml) and brine (1x 15 ml). After drying over Na2SO4, the volatiles were removed on a rotary evaporator to yield the amine (for yields see Table 1).
All amines were purified by column chromatography.
The reaction does not beat the reduction with Al/Hg but it is none the less an alternative, not using toxic reagents.
Reduction of carboxylic acid ester to alcohols with PMHS and a catalyst
More interesting is the reduction of benzoic acid ethyl ester with PMHS with potassium fluoride (KF) to benzyl alcohol in DMSO (yield: 81%, purity: 90%) described in
Synthesis, 1982, 981 (http://www.angelfire.lycos/scifi2/lego/
DOI:10.1055/s-1982-30035
A similiar method is described in
Synlett, 1997, 989-991 (http://www.angelfire.lycos.com/scifi2/l
DOI:10.1055/s-1997-951
but this time with PMHS and catalytic amounts of tetrabutylammonium fluoride (TBAF) or benzyltrimethylammonium hydroxide (Triton® B).
Table 1. Yields for the reduction of substituted esters 4 and acids 5
| R | Yield (%) 1 --> 2 (R1 = OMe) |
Yield (%) 3 --> 2 (R1 = OH) |
| H | 95 | 82 |
| 2,4-Cl2 | 94 | 69 |
| 2,4-Me2 | 93 | 72 |
| 4-Me | 93 | 70 |
| 3,5-Cl2 | 83 | 75 |
| 4-Br | 96 | 74 |
| 4-Cl | 97 | 67 |
| 3,4,5-OMe3 | 81 | 79 |
| 3-Me | 90 | 78 |
Scheme 2. Reduction of esters with PMHS/TBAF (cat.)
| Phenylacetic acid methyl ester | Phenylethanol | 76% |
| Cinnamic acid methyl ester | Cinnamyl alcohol | 85% 1,2:1,4 99:1 |
| CH2=CH-(CH2)8COOCH3 | CH2CH-(CH2CH2OH | 95% |
| R-Mandelic acid methyl ester | R-Phenylethandiol | 90%, e.e. >95% |
(Un)substituted acetophenones, benzaldehydes and ketones are also reduced in good yields to alcohols.
The first step of the reduction of acids is the formation of a silylester, resulting in the consumption of 1 equivalent of silane. Indeed, the hydrogen liberated is seen bubbling from the reaction mixture immediately after addition of TBAF. Care must be taken when carrying out the reduction on large scale to ensure that the hydrogen liberated is safely vented from the reaction. The initial product of the reaction is a silylether and is cleaved to to the alcohol by treatment with aqueous sodium hydroxide. However, for base-sensitive substrates the silylether may be cleaved by refluxing in aqueous methanol (8 h). In this case addition of extra fluoride (10 mol%) aids the reaction. The silyl ether is also cleaved by stirring with aqueous hydrochloric acid (1N, r.t., 1.5 h). Aliphatic esters are cleaved without complication.
[...]
Triton® B does not promote the reduction of esters with PMHS; it seems that the OH group facilitates the cross-linking of the polymer faster than the reduction process.
[...]
No explicit details are given for the reduction of carboxylic acids
Standard procedure
To a stirred mixture of ester/ketone/aldehyde (1 mmol) and tetrabutylammonium fluoride or Triton® B (0.02 mmol) in dry tetrahydrofuran (2 ml) was added polymethylhydrosiloxan (Lancaster)(1.5 mmol for the reduction of ketones and aldehydes; 3 mmol for the reduction of esters). The mixture was stirred at room temperature until the reaction was complete (by t.l.c). Sodium hydroxide (5 ml of a 3N solution) was added dropwise. After stirring vigorously overnight the solution was extracted with diethyl ether (3 x 15 ml). The combined organic extracts were washed with water, dried (MgSO4) and evaporated in vacuo. The residue was purified by chromatography (SiO2) or destillation if necessary.
Reduction of carboxylic esters and acids by polymethylhydrosiloxane catalysed by titanium and zirconium
Synlett, 1994, 10, 833-835 (http://www.angelfire.lycos.com/scifi2/l
DOI:10.1055/s-1994-23022
1: R-COOMe
2: R-CH2OH
Table 1. Reduction of methyl esters 1 with PMHS and Ti(OiPr)4
| R | Molar ratio 1:PMHS | Molar ratio 1:Ti(OiPr)4 | Time (h) | Yield (%) of 2 |
| Ph | 10 | 1 | 5 | 86 |
| PhCH2 | 10 | 1 | 8 | 76 |
| PhCH2CH2 | 10 | 1 | 16 | 65 |
| E-PhCH=CH | 10 | 1 | 5 | 82 |
| p-NO2-C6H4 | 10 | 1 | 16 | 84 |
| p-HO-C6H4 | 10 | 1 | 16 | 81 |
| p-MeO-C6H4 | 10 | 1 | 16 | 88 |
| Me(CH2)10 | 10 | 1 | 16 | 89 |
| Me(CH2)16 | 10 | 1 | 16 | 98 |
| Me(CH2)16 | 5 | 0.5 | 16 | 93 |
| Me(CH2)16 | 2 | 1 | 16 | 92 |
| Me(CH2)16 | 10 | 0.5 | 80 | 87 |
7: R-COOH
Table 2 Reduction of carboxylic acids 7 with PMHS and Ti(OiPr)4
| R | Molar ratio 7:PMHS | Molar ration 7:Ti(OiPr)4 | Time (h) | Yieds (%) of 2 |
| Me(CH2)16 | 10 | 1 | 16 | 86 |
| Ph | 10 | 1 | 16 | 69 |
| p-NO2--C6H4 | 10 | 1 | 16 | 76 |
| p-Me-C6H4 | 10 | 1 | 30 | 70 |
| p-MeO-C6H4 | 10 | 1 | 48 | 88 |
| o-MeO-C6H4 | 10 | 1 | 30 | 63 |
Standard procedure
To a stirred mixture of ester (1 mmol) and polymethylhydrosiloxane (10 mmol) in tetrahydrofuran (AR grade) (2 ml) was added titantium (IV) isopropoxide (1 mmol). The mixture was heated under reflux with an outlet to a nitrogen line until all the ester has been consumed (as jugded by tlc). The solution was cooled and sodium hydroxide solution (7 ml of a 3 N solution) was carefully aded dropwise (at first there is vigorous gas evolution). After stirring vigorously overnight the solution is extracted with ether (3 x 10 ml). The ether layers were washed with hydrochloric acid (25 ml of a 1 M solution), dried (Na2SO4) and evaporated in vacuo to give the alcohol in essentially pure form.
Safety note
Buchwald reports that in the absence of substrate the system generates a highly dangerous gas, possibly silane, SiH4[/sup]16[/sup]. Although they had no problems with the system, like ourselves, there has been a report of an explosion using triethoxysilane reagent system17. We therefore advise that appropriate precautions be taken to avoid possible build-up of large quantities of silane, when using this protocol.
16. Can. J. Chem., 1990, 68, 471
17. J. Org. Chem., 1993, 58, 3221
These reductions mighte bee useful for the reduction of gallic acid (3,4,5-trihydroxybenzoic acid) as a precursor for mescaline and 2,5-dihydroxybenzoic acid for the 2C-X series.
All italic text by Lego
Please post all other methods involving PMHS in this thread.
The candle that burns twice as bright burns half as long
(Chief Bee)
07-10-03 02:03
No 445977
My contribution is that I have now uploaded and added a link to the full-text article abstracted in Post 170334 (Lilienthal: "Novel reductive amination with PMHS-Ti(OiPr)4", Novel Discourse).
(Hive Bee)
07-12-03 18:39
No 446637
Perhaps this should have been placed here
Post 446608 (java: "Direct Zn–diamine promoted reduction of CO and CN", Chemistry Discourse)since it deals with PMHS but being more recent approach with only methanol and Zn-diamine , I only hope someone will get the full text....java
We're all in this world together,
http://www.chiapaslink.ukgateway.net/
(Chief Bee)
07-13-03 00:56
No 446702
(Rated as: good read)
Direct Zn–diamine promoted reduction of C=O and C=N bonds by polymethylhydrosiloxane in methanol
Virginie Bette , André Mortreux , Christian W. Lehmann and Jean-François Carpentier
Chem. Commun., (3), 332-333 (2003) (../rhodium/pdf /pmhs.imine2a
DOI:10.1039/b210144k
Abstract
Ketones and imines are chemoselectively reduced at room temperature in methanol to the corresponding alcohols and amines in high yields in a one-step procedure using polymethylhydrosiloxane (PMHS) and a simple zinc–diamine catalyst.
(Hive Bee)
05-05-04 20:01
No 505083
(Rated as: good read)
Identification of new catalysts for the asymmetric reduction of imines into chiral amines with polymethylhydrosiloxane using high-throughput screening
Tania Ireland, Francois Fontanet and Guen-Gnanh Tchao
Tet. Lett., 2004, 45, in press
DOI:10.1016/j.tetlet.2004.03.159

Abstract
The use of high-throughput techniques allowed the rapid identification of new catalysts for the enantioselective reduction of imines using polymethylhydrosiloxane (PMHS) as a reducing agent. By a simple modification of the chiral ligand structure that came out of the screening, the enantioselectivity of the reduction was increased from 40% ee to 60% ee.
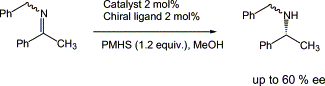
The tendency is to push it as far as you can
(Chief Bee)
09-05-04 10:54
No 529706
(Rated as: excellent)
Rapid Defunctionalization of Carbonyl Group to Methylene with Polymethylhydrosiloxane-B(C6F5)3
S. Chandrasekhar, Ch. Raji Reddy, and B. Nagendra Babu, J. Org. Chem. 67, 9080-9082 (2002)
Abstract
The polymethylhydrosiloxane-B(C6F5)3 combination is found to be a versatile carbonyl defunctionalization system under mild and rapid conditions. For the first time, B(C6F5)3 has been used as a nonconventional Lewis acid catalyst to activate PMHS. Aromatic and aliphatic carbonyl compounds were effectively reduced to give the corresponding alkanes in high yields.
Defunctionalization of organic functional groups is an equally desirable achievement as compared to functionalization. There is a great need to discover new methodologies for defunctionalization especially for conversion of polyfunctional natural products to useful building blocks and bioactive molecules. Available literature speaks of only a few protocols for removal of a certain functional group, viz., the carbonyl group can be defunctionalized to a methylene group by Clemensen1 or Wolff-Kishner reduction,2 both of which require very drastic reaction conditions. The hydroxyl group can be removed by a Barton-McCombie procedure,3 wherein highly malodorous xanthate and Bu3SnH are required. Some other methods known in the literature include catalytic hydrogenation4 and reaction involving use of PtO2,5 HI-phosphorus,6 BH3,7 Zn/HCl/HgCl2/H2O,8 NaBH4-CF3CO2H,9 NaCNBH3-BF3Et2O,10 LAH-AlCl3,11 Et3SiH-BF3Et2O or CF3CO2H12 besides a few others.13 The majority of the known procedures used for defunctionalization are nonchemoselective and require harsh reaction conditions.
All of these methods, while offering some advantages, also suffer from disadvantages. Most of these methods are generally restricted to aromatic systems, are some times harsh and need a pyrophoric hydride source for reduction, require longer reaction hours with careful workup procedures for quenching the excess reagent, and are often associated with low yields. The usefulness of polymeric hydride source polymethylhydrosiloxane (PMHS), a coproduct of the silicone industry, as an excellent reduction reagent is well demonstrated in several recent publications.14,15 The quest to find newer activators for this rather inert polymer resulted in identification of tris(pentafluorophenyl)borane as an excellent catalyst for activation of PMHS. B(C6F5)316 is a relatively unexplored Lewis acid. This combination of PMHS-B(C6F5)3 is found to be a versatile carbonyl defunctionalization system with very short reaction times (Scheme 1). Interestingly, this combination establishes a powerful "catalytic switch", viz., our initial studies using ZnCl2 as an activator resulted in reduction of ketone to alcohol,17 whereas this new catalyst promoted the reduction of the same substrate to methylene group.18 The procedure is very simple, and the reaction completion is indicated by the termination of effervescence (reaction times ranging from 5 to 20 min).
Scheme 1
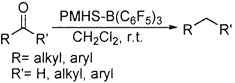
The earlier procedures involving PMHS as a hydride source for similar transformations required stoichiometric amounts of AlCl3 as an activator13d and were limited to aryl carbonyl compounds, whereas in the case of Pd/C as an activator,13f double bonds are reduced to saturation instead of carbonyl reduction to a methylene group.
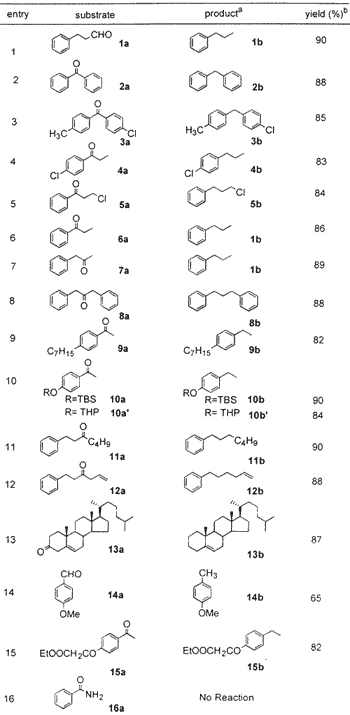
To establish the optimum reaction conditions, the reaction was first studied on readily available benzophenone 2a, which was reduced to diphenylmethane 2b in 88% isolated yield (entry 2) in 10 min. Another substrate, phenyl propanaldehyde 1a, was reduced to n-propyl benzene 1b in 90% yield (entry 1) in 8 min. These two examples demonstrate that not only benzylic ketone (a very easily reducible carbonyl) but also aliphatic aldehyde is suitable for the present protocol (Table 1). The halo-substituted aryl ketones 3a, 4a, and 5a also were reduced to the corresponding methylene compounds 3b, 4b, and 5b without affecting the aryl halide group (entries 3 and 4). The alkyl halide group (entry 5) is also unaffected under the present protocol. Aliphatic keto substrates 7a, 8a, 11a, 12a, and 13a were also well suited to the present reaction conditions (entries 7, 8, and 11-13). Substrates 10a and 10a' demonstrate the selective reduction of ketone in the presence of TBS and THP ethers (entry 10), and substrate 12a demonstrates inertness to olefin functionality (entry 12). The steroid substrate 13a was reduced without isomerization of double bond in 87% yield requiring only 10 min (entry 13). The reduction of anisaldehyde 14a to 4-methyl anisole 14b was also achieved, albeit in low yield (65%). In the case of compound 15a (entry 15), where both carbonyl and ester groups are present in the same substrate, selective reduction of carbonyl group to methylene is observed in 82% isolated yield. Attempts, however, to reduce benzamide 16a to benzylamine (entry 16) were futile.
Hypothetically, we propose that complex C, which is formed from B(C6F5)3 A and PMHS B, is responsible for the reduction of the carbonyl functionality.16a Complex C would react with carbonyl group D to form E (not isolated), which would produce the reaction product, hydrocarbon F, and silyl ether G and would regenerate A (Scheme 2). Coordination of carbonyl oxygen to boron for facile hydride transfer is also expected. There is literature precedence that Ph3SiH and Et3SiH also operate in a more or less similar pathway; the intermediate E is isolable when 1 equiv of Ph3SiH is used,16b and longer reaction times (20 h) are required when Et3SiH is used.16c However, in the case of PMHS, even though 1 equiv of reagent is used, the intermediate E could not be isolated, and instead 45-50% hydrocarbon conversion was observed within 5-20 min and the remaining starting carbonyl was isolated. This clearly indicates that PMHS is a more powerful reducing agent than Ph3SiH and Et3SiH in the presence of B(C6F5)3.
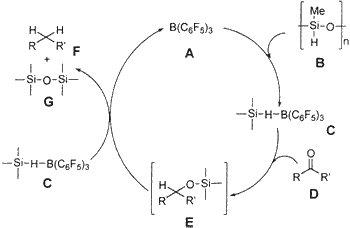
Scheme 2
In Summary, we have demonstrated for the first time, a direct and rapid conversion of carbonyl functionality to methylene group under very mild conditions with high yields. The procedure is very simple, and progress of the reaction can be monitored by visualization without any analytical support. The shorter reaction time in all the cases studied is an added advantage.
Experimental Section
General Methods
Silica gel used was 60-120 mesh. 1H NMR spectra were obtained in CDCl3 at 200 MHz. Chemical shifts are given in parts per million with respect to internal TMS, and J values are given in hertz. Methylene chloride was distilled over CaH2 prior to use.
General Procedure for Defunctionalization of Carbonyl Group with PMHS-B(C6F5)3
To a solution of carbonyl compound (1 mmol) in dry CH2Cl2 (5 mL) and tris(pentafluorophenyl)borane (5 mol%) was slowly added polymethylhydrosiloxane (3 mmol) at room temperature. After 5-20 min, a vigorous effervescence (like foam) was observed. At this point, the solvent was evaporated and reaction mixture was dissolved in hexane and filtered through a silica gel pad using hexane. Evaporation of the volatiles afforded the reduction product in pure form. Spectral data of all products other than 9b, 10b, and 10b' were identical with those of authentic samples.19
Table 1
References
1.(a) Clemmensen, E. Chem. Ber. 1914, 47, 51, 681. (b) Vedejs, E. Org. React. 1975, 22, 401.
2. (a) Kishner, J. J. Russ. Phys. Chem. Soc. 1911, 43, 582. (b) Wolff, C. Liebigs Ann. 1912, 394, 86. (c) Todd, D. Org. React. 1948, 4, 378. (d) Minlon, H. J. Am. Chem. Soc. 1949, 71, 3301.
3. (a) Barton, D. H. R.; McCombie, S. W. J. Chem. Soc., Perkin Trans 1 1975, 1574. (b) Barton, D. H. R. Tetrahedron 1986, 42, 2329.
4. (a) Lee, W. Y.; Park, C. H.; Kim, H. J.; Kim, S. J. Org. Chem. 1994, 59, 878. (b) Lee, W. Y.; Park, C. H.; Kim, Y. D. J. Org. Chem. 1992, 57, 4074.
5. Rao, A. V. R.; Mahendale, A. R.; Reddy, K. B. Tetrahedron Lett. 1982, 23, 2415.
6. (a) See ref 4b. (b) Reimschneider, R.; Kassahn, H. Chem. Ber. 1959, 92, 1705. (c) Bradsher, C.; Vingiello, F. J. Org. Chem. 1948, 13, 786.
7. (a) Kelly, T. R.; Kim, M. H. J. Am. Chem. Soc. 1994, 116, 7072. (b) Breuer, E. Tetrahedron Lett. 1967, 20, 1849.
8. Lee, W. Y.; Park, C. H.; Kim, E. H. J. Org. Chem. 1994, 59, 4495.
9. (a) Ketcha, D. M.; Lieurance, B. A.; Homan, D. F. J. J. Org. Chem. 1989, 54, 4350. (b) Gribble, G. W.; Kelly, W. J.; Emery, S. E. Synthesis 1978, 763.
10. Srikrishna, A.; Viswajanani, R.; Sattigeri, J. A.; Yelamaggad, C. V. Tetrahedron Lett. 1995, 36, 2347.
11. (a) Paquette, L. A.; Maleczka, R. E., Jr. J. Org. Chem. 1992, 57, 7118. (b) Blackwell, J.; Hickinbottom, W. J. J. Chem. Soc. 1961, 1405.
12. (a) Smonou, I. Tetrahedron Lett. 1994, 35, 2071. (b) Fry, J. L.; Orfanopoulos, M.; Adlington, M. G.; Dittman, W. P.; Silverman, S. B. J. Org. Chem. 1978, 43, 374. (c) West, C. T.; Donelly, S. J.; Kooistra, D. A.; Doyle, M. P. J. Org. Chem. 1973, 38, 2675. (d) Smith, C. N.; Ambler, S. J.; Steggler, D. J. Tetrahedron Lett. 1993, 34, 7447. (e) Kursanov, D. N.; Parnes, Z. N.; Loim, N. M. Synthesis 1974, 633.
13. (a) Brieger, G.; Fu, T.-H. J. Chem. Soc., Chem. Commun. 1976, 757. (b) Karaman, R.; Fry, J. L. Tetrahedron Lett. 1989, 30, 4931. (c) Ram, S.; Spicer, L. D. Tetrahedron Lett. 1988, 29, 3741. (d) Jaxa-Chamiec, A.; Shah, V. P.; Kruse, L. I. J. Chem. Soc., Perkin Trans. 1 1989, 1705. (e) Larock, R. C. Comprehensive Organic Transformations, 2nd ed.; Wiley & Sons: 1999, p 61. (f) Lipowitz, J.; Bowman, S. A. J. Org. Chem. 1973, 38, 162.
14. (a) Chandrasekhar, S.; Reddy, Ch. R.; Rao, R. J.; Rao, J. M. SynLett 2002, 349. (b) Chandrasekhar, S.; Reddy, Ch. R.; Rao, R. J. SynLett 2001, 1561 and references therein.
15. For an exhaustive review on PMHS, see: (a) Lawrence, N. J.; Drew, M. D.; Bushell, S. M. J. Chem. Soc., Perkin Trans. 1 1999, 3381. Also see: (b) Nitzsche, S.; Wick, M. Angew. Chem. 1957, 69, 96. (c) Mimoun, H.; Laumer, J. Y. S.; Giannini, L.; Scopelliti, R.; Floriani, C. J. Am. Chem. Soc. 1999, 121, 6158. (d) Verdaguer, X.; Lange, U. E. W.; Buchwald, S. L. Angew. Chem., Int. Ed. 1998, 37, 1103. (e) Lopez, R. M.; Fu, G. C. Tetrahedron 1997, 53, 16349 (f) Breeden, S. W.; Lawrence, N. J. Synlett 1994, 833. (g) Drew, M. D.; Lawrence, N. J.; Fontaine, D.; Sehkri, L. Synlett 1997, 989. (h) Mimoun, H. J. Org. Chem. 1999, 64, 2582 and references therein.
16. (a) Gevorgyan, V.; Rubin, M.; Benson, S.; Liu, J.-X.; Yamamoto, Y. J. Org. Chem. 2000, 65, 6179. (b) Parks, D. J.; Piers, W. E. J. Am. Chem. Soc., 1996, 118, 9440. (c) Gevorgyan, V.; Rubin, M.; Liu, J.-X.; Yamamoto, Y. J. Org. Chem. 2001, 66, 1672. (d) Parks, D. J.; Blackwell, J. M.; Piers, W. E. J. Org. Chem. 2000, 65, 3090.
17. Chandrasekhar, S.; Reddy, Y. R.; Ramarao, C. Synth. Commun. 1997, 27, 2251.
18. Polymethylhydrosiloxane in the presence of AlCl3 reduced aryl carbonyl group to methylene; see ref 13d.
19. All authentic samples are commercially available except 3b, 4b, and 12b. For 3b, see: (a) Fukuzawa, S.; Tsuchimoto, T.; Hiyama, T. J. Org. Chem. 1997, 67, 151. 4b: (b) Khosrovi, M.; Partchamazad, I.; Fakhrai, H. Tetrahedron Lett. 1975, 16, 2619. 12b: (c) Gamage, S. A.; Smith, R. A. J. Tetrahedron 1990, 46, 2111.
The Hive - Clandestine Chemists Without Borders