(Hive Bee)
07-10-03 03:26
No 446144
(Rated as: excellent)
Iron-mediated aromatic allylation
Tetrahedron Letters, Vol. 28, No. 45, pp 5415-5418 (1987)
Janice W. Dieter,a Zhong Lib and Kenneth M. Nicholasb*
Departments of Chemistry, Boston College,a Chestnut Hill, MA 02167 and University of Oklahoma, b Norman, OK 73019(USA)
DOI:10.1016/S0040-4039(00)96742-X
pdf: http://mishmashblue.tripod.com/tlallylat
TFSE additional keyword: gamma-asarone
n = eta
Abstract:
Electron rich aromatic and heteroaromatic compounds react with (n3-allyl)Fe(CO)4BF4 to produce allylated aromatics in moderate to good yields. Unsymmetrically substituted allyl complexes afford the corresponding butenyl-,1,1-dimethylallyl-, cinnamyl-, and geranyl-derivatives with moderate to excellent regioselectivity and complete stereoselectivity.
The widespread natural occurrence of allyl and isoprenyl aromatic compounds1 has promoted the long standing interest in synthetic methods for introduction of these side chains onto the aromatic nucleus. The classical methods of Friedel-Crafts alkylation2 and Claisen rearrangement,3 and the available organometallic couplings (M = Mg,4 Ni,5 Si6) variously suffer from limited chemo-, regio-, and stereo-selectivity with respect to both the aromatic, and, especially, the allyl components. Prompted by prior reports of the attack of (n3-allyl)Fe(CO)4+ species by various heteroatomic nucleophiles and stabilized enolates,7a-c we have examined the reactivity of these complexes towards aromatics in an effort to overcome the above limitations of aryl/allyl coupling. Herein we report that such reactions provide a convenient method for aromatic allylation, with moderate to excellent regio- and stereocontrol.
Whereas nitromethane solutions of the cations 1a-e under 1 atm CO were unreactive at 20°C towards mildly activated aromatics such as anisole, phenol, p-hydroquinone, and 2-methyl-1,4-dimethoxynaphthalene, more electron rich substrates including N,N-dimethylaniline, pyrrole, 1 ,3-C6H4(OMe)2, 1,2,4-C6H3(OMe)3, 1,3,5-C6H3(OMe)3, furan and 2-methylindole underwent ready reaction as determined by IR and GC monitoring (eq. 1).
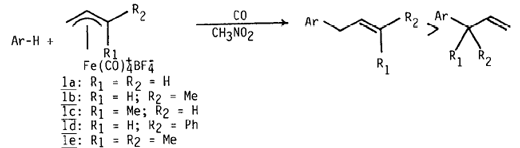
Upon reaction completion, the mixture was diluted with ether and washed with brine. The organic phase was treated with iodine, washed with aqueous thiosulfate, dried, and the products were isolated by distillation, flash chromatography on silica gel, or preparative GC (to separate isomers). Structures were established on the basis of combined IR, 1H NMR (300 MHz) and mass spectral data; new compounds gave satisfactory elemental analyses or high resolution mass spectra. Results are summarized in the Table.
In all cases examined the observed aromatic regioselectivity is that expected based on attack by the allyl complexes at the site of greatest combined electrophilicity/accessibility. In several instances, especially with the parent complex 1a, 1:1 substrate/complex reactant ratios afforded small amounts of dialkylated products (ca. 10%) which could be almost entirely suppressed when 1 was added slowly to a modest excess of the substrate.
The regio- and stereoselectivity of coupling with respect to the allyl fragment was probed using the substituted allyl complexes 1b-e. These were conveniently prepared by in situ protonation of the corresponding (n2-allyl alcohol)Fe(CO)4 species.8 The syn- and anti-(n3-methallyl)Fe(CO)4+ complexes, 1b and 1c, reacted with 1,2,4-trimethoxybenzene (1,2,4-TMB) with moderate selectivity for attack at the less-substituted allyl terminus but with essentially complete stereoselective retention of the allyl fragment’s geometry, consistent with our recent observations involving reactions of 1b,c with stabilized enolates.7c On the other hand, the syn-cinnamyl derivative 1d and 1,2,4-TMB combined regio- and stereospecifically (attack at less-substituted terminus and retention of allyl geometry) affording violastyrene methyl ether as the only detectable product. The 3,3-dimethylallyl chain, so prevalent in naturally occurring aromatic compounds, was specifically introduced into 1,3,5-TMB and methylindole using complex 1e under the standard conditions. These findings are particularly significant in light of the inefficacy of the Friedel-Crafts and Claisen approaches to this unit. 2,3
An initial assessment of the prospects for introduction of polyisoprenyl side chains using this method also was very encouraging. Although linalool (2) formed an unstable, difficult to isolate n3-Fe(CO)4+ derivative under typical conditions,8 generation of the neutral (n2-linalool)Fe(CO)4 complex (Et2O, 1 atm CO, 25°C, 8 h) followed by its dissolution in CH3NO2 and addition of 1,3,5-TMB (0.6 equiv) and HBF4/Ac2O (1 equiv) led to the completely stereoselective formation of 1-geranyl-2,4,6-C6H2(OMe)39 in 63% yield along with a small amount (ca 10%) of an unidentified, apparently isomeric product (eq. 2).
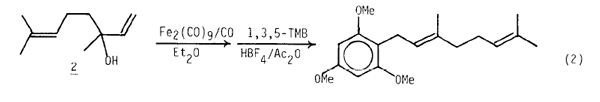
This intriguing result, which presumably proceeds via a transient (n3-geranyl)Fe(CO)4+ complex, should be contrasted with coupling reactions of the corresponding (n3-allyl)Ni-species5 which give E,Z isomer mixtures.
Efforts are underway to further establish the scope of these coupling reactions and to illustrate their utility in the synthesis of naturally occurring polyisoprenoid derivatives.
Table: Aromatic allylation with (n3-allyl)Fe(CO)4BF4, 1a-ea
| Complex | Substrate | Substrate/Complex | Time (hr) | Products | Yield (%)b |
| 1a | 1,3-dimethoxybenzene | 1:1 | 20 | 1-allyl-2,4-dimethoxybenzene | 60 |
| 1,2,4-trimethoxybenzene | 1:1 | 20 | 1-Allyl-2,4,5-trimethoxybenzene | 87 | |
| 1,3,5-trimethoxybenzene | 1.5:1 | 4 | 1-Allyl-2,4,6-trimethoxybenzene | c | |
| 1,3,5-trimethoxybenzene | 1:1 | 4 | " + 10% diallylated | 79 | |
| 2-Methyl-1H-indole | 2:1 | 2d | 3-Allyl-2-methyl-1H-indole | c | |
| 2-Methyl-1H-indole | 1:1 | 2d | " + 11% 3,3 and 1,3-diallylated | 81 | |
| Furan | ~25:1 | 2 | 2-Allylfuran | 31 | |
| 1b | 1,2,4-trimethoxybenzene | 1:1 | 24 | trans1-But-2-enyl-2,4,5-trimethoxy-benzene (3:1)e 1,2,4-Trimethoxy-5-(1-methyl-allyl)-benz |
68 |
| 1c | 1,2,4-trimethoxybenzene | 1:1 | 24 | cis1-But-2-enyl-2,4,5-trimethoxy-benzene (4:1)e 1,2,4-Trimethoxy-5-(1-methyl-allyl)-benz |
61 |
| 1d | 1,2,4-trimethoxybenzene | 1:1 | 24 | 1-(3-phenyl-allyl)-2,4,5-trimethoxy-benz e |
60 |
| 1e | 1,3,5-trimethoxybenzene | 1:1.5 | 4 | 1,3,5-Trimethoxy-2-(3-methyl-but-2-enyl) (>50:1) 2-(1,1-Dimethyl-allyl)-1,3,5-trime |
51 |
| 2-Methyl-1H-indole | 1:1.2 | 2d | 2-Methyl-3-(3-methyl-but-2-enyl)-1H-indo (>50:1)f 3-(1,1-Dimethyl-allyl)-2-methyl-1H-indol |
73 |
a Reactions run at 25°C in CH3NO2 unless noted otherwise;
b non-optimized, isolated yield;
c yield not determined;
d T=0°C;
e <5% stereoisomer detected by GC;
f ca. 10% dialkylation.
References:
1 T.K. Devon and A.I. Scott in "Handbook of Naturally Occurring Compounds," Vol. I,
Academic Press, NY (1975).
2 R. Koncos and B.S. Friedman, "Friedel-Crafts and Related Reactions" ed. by G.A. Olah,
Vol. II, Part II, pp 289-412, Wiley, N.Y. (1964); C.C. Price, "Organic Reactions," ed.
by R. Adams, Vol. 3, pp 1-82, Wiley, N.Y. (1946); D.E. Wolf, C.H. Hoffman, N.R. Trenner,
B.H. Arison, C.H. Shunk, B.O. Lin, J.F. McPherson and K. Folkers, J. Am. Chem. 1958, 80, 4752;
U. Gloor, O. Isler, R.A. Morton, R. Ruegg and O. Wiss, Helv. Chim. Acta,1958, 5, 2357.
3 D.S. Tarbell, "Organic Reactions," ed. by R. Adams, Vol. 2, pp l-48, Wiley, N.Y. (1944).
4 S. Suzuki, M. Shiono and Y. Fujita, Synthesis, 804, 1983.
5 K. Sato, S. Inoue and K. Saito, J. Chem. Sot. Perkin I, 1973, 2289;
L.S. Hegedus, E.L. Waterman and J. Catlin, J. Am. Chem. Soc., 1972, 94, 7155.
6 M. Ochiai, E. Fujita, M. Arimoto and H. Yamaguchi, Chem. Pharm. Bull., 1985, 33, 41;
ibid, 1982, 30, 3994; ibid, 1983, 31, 86.
7 a) T.H. Whitesides, R.W. Arhardt, R.W. Slaven, J. Am. Chem. Soc., 1973, 95, 5793;
b)A.J. Pearson, Tetrahedron Lett., 1975, 3617;
c) C.S. Silverman, S. Strickland and K.M. Nicholas, Organometallics, 1986, 5, 2117.
8 J. Dieter and K.M. Nicholas, J. Organometal. Chem., 1981, 212, 107.
Structural assignment supported by homonuclear decoupling and NOE difference
experiments.
Got democracy?http://www.dhushara.com/book/multinet/de