(Hive Bee)
07-31-03 23:21
No 450943
(Rated as: excellent)
Note of caution: The halides of phosphorus are seriously hazardous to one's health and special precautions have to be taken when handling these compounds.
Material Safety Data Sheet:
Phosphorus trichloride (PCl3) (http://physchem.ox.ac.uk/MSDS/PH/phosph
Phosphorus pentachloride (PCl5) (http://physchem.ox.ac.uk/MSDS/PH/phosph
Phosphorus oxychloride (POCl3) (http://physchem.ox.ac.uk/MSDS/PH/phosph
taken from Mellor's modern inorganic chemistry, new revised edition 1963, p829-833 :
Phosphorus forms two series of compounds with the halogens, represented by the general formulae PX3 and PX5. It is doubtful if the penta-iodide has been obtained, but otherwise all the members of both series are known. The oxyhalides POX3 are also known.
Phosphorous trichloride
This compound can be made by the action of dry chlorine on yellow phosphorus in the following apparatus :
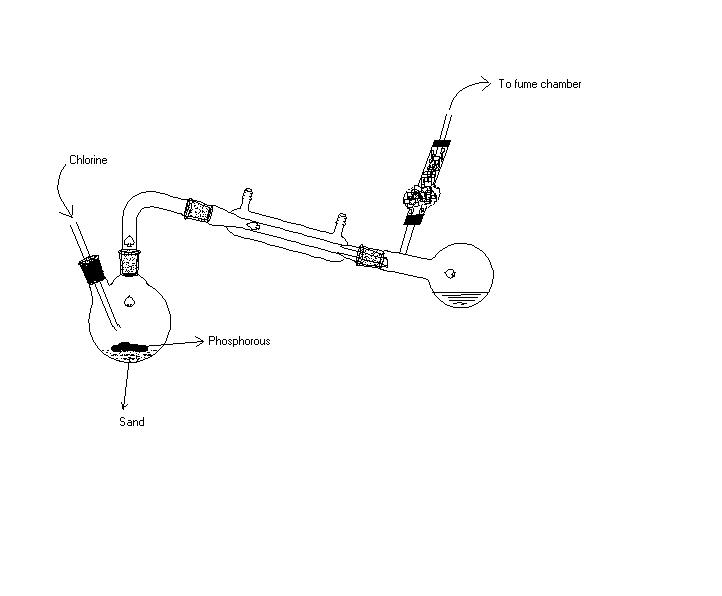
I found no retort pic in Chemdraw so I used above modification
This consists of a retort containing a layer of sand on which rests a quantity of dry yellow phosphorus, the air having been displaced by carbon dioxide. The retort is heated gently. Chlorine, dried by sulphuric acid, is passed in by means of a movable tube. The distance of this tube is so arranged that the phosphorus does not distill (which occurs if it is too near), neither is the pentachloride formed (which happens if it is too far off). When the action has begun, a tongue of flame projects from the chlorine tube, and the retort needs no further heating
Phosphorus trichloride is a mobile liquid with an unpleasant smell. It boils at 73.5°, fumes in air and is hydrolysed by water, forming phosphorous acid and hydrochloric acid:
It can be frozen to a solid which melts at -111.8°.
Phosphorus pentachloride
PCl5 is best prepared by the action of an excess of chlorine on the trichloride. It is convenient to prepare it in the vessel in which it is to be stored.
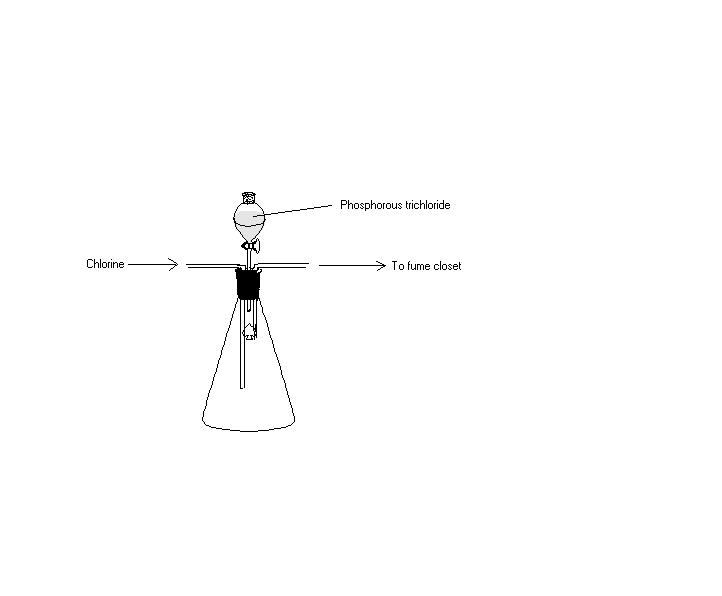
Excuses for the crappy drawing.
PCl3 + Cl2 __> PCl5
It can also be made by the direct action of chlorine on yellow phosphorus ; or by the action of sulphur chloride on phosphorus trichloride :
Phosphorus pentoxide when pure is an almost colourless solid; as obtained it is usually pale greenish-yellow. It sublimes below 100°, but if heated under pressure melts at 148°C. It reacts violently with water, forming first the oxychloride, and then orthophosphoric acid:
POCl3 + 3H2O __> H3PO4 + 3HCl
Like phosphorus trichloride, it is a valuable reagent for transforming hydroxyl compounds into chlorides, and finds extensive use for this purpose in organic chemistry.
The vapour density of phosphorus pentachloride varies with temperature. The following values were obtained by J.B. Dumas:
| Temperature | 182° | 200° | 250° | 300° |
| Vapour density | 73.3 | 70 | 57.6 | 52.4 |
The theoretical value for PCl5 is 104.25 (H2 = 1). It is inferred, therefore, that dissociation is taking place into free chlorine and phosphorus trichloride :
This has been confirmed experimentally by the facts that starch potassium iodide paper give the characteristic blue coloration produced by chlorine when immersed in the vapour of phosphorus pentachloride; and that the addition of chlorine reduces the amount of dissociation as would be expected from the application of the Law of Mass Action to the equilibrium. Thus, with the notation previously employed we have:
---------- = K
[PCl3][Cl2]
Consequently, if the concentration of chlorine or of phosphorus trichloride be raised, by addition of one or other to the system in equilibrium, some combination will take place and the concentration of the pentachloride will thereby increased. That is, dissociation will be reduced.
Phosphoryl Chloride, Phosphorus Oxychloride, POCl3
This compound can be made by very carefully adding water to phosphorus pentachloride until the solid disappears:
It is also made by the gradual addition of powdered potassium chlorate to phosphorus trichloride at ordinary temperatures, and distillation of the mixture :
It is now manufactured from finely ground phosphate rock which is mixed with activated coke and then treated with chlorine (sometimes mixed with carbon monoxide) at 400°.
Ca3(PO4)2 + 6Cl2 + 6CO __> 2POCl3 + 3CaCl2 + 6CO2
Phosphorus oxychloride is a colourless fuming liquid which boils at 107.2° and can be solidified to a colourless crystalline mass melting at -1.25°. In aqueous solution it is slowly hydrolysed to phosphoric and hydrochloric acids.
Kluwer's Chemisch-technisch Handboek, 1st print 1954 has the following information:
PCl3: mol wt. 137.35, mp -91°, bp 76°
Decomposed by water and alcohol. Soluble in benzene, CS2 and ether. Made by "burning" phosphorus in a stream of dry chlorine, a yellow flame is observed.
PCl5: mol wt 208.27, sublimes at 162°.
Decomposed by water and alcohol. Soluble in carbon tetrachloride and CS2. Prepared by dissolving 150 g white phosphorus in 600 mL CS2 and bubble absolutely dry chlorine in until the solution is totally saturated with chlorine. PCl5 will crystallise out. The carbon disulfide is distilled off on a water bath.
POCl3: mol wt 153.35, mp 2°, bp 105.3°
Decomposed by water and alcohol. Prepared by the dropwise addition of PCl3 to absolutely dry potassium chlorate.To avoid explosions one adds some pre-made POCl3 to the KClO3. Also from PCl3 and SO2Cl2, giving thionyl chloride as a side-product.
A Dream Within A Dream (http://www.poedecoder.com/Qrisse/works/
(Newbee)
08-29-03 18:58
No 456359
Great post man.
Thanks.
Using these compounds in synthesis is not popularized here, but what a shame.
(Hive Bee)
09-02-03 03:22
No 456789
Preparing and handling these compounds is not something for the novice or less-equipped chemist to perform.
I guess for many bees cyanuric chloride holds more potential as a safer to handle, unwatched chlorination agent.
A Dream Within A Dream (http://www.poedecoder.com/Qrisse/works/