08-14-03 03:33
No 453444
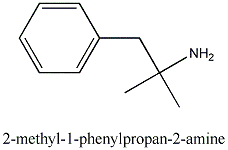
Is anything known about this species?
(Hive Bee)
08-14-03 03:38
No 453445
It's called Phentermine.
fear fear hate hate
(Stranger)
08-14-03 04:29
No 453452
I just thought phentermine looked like
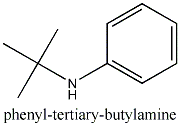
Thanks, now I know what to UTSFE for.
(Hive Bee)
08-14-03 10:19
No 453490
Monograph Number: 7346
Title: Phentermine
CAS Registry Number: 122-09-8
CAS Name: a,a-Dimethylbenzeneethanamine
Additional Names: a,a-dimethylphenethylamine; phenyl-tert-butylamine; a-benzylisopropylamine
Molecular Formula: C10H15N
Molecular Weight: 149.23.
Percent Composition: C 80.48%, H 10.13%, N 9.39%
Literature References: Prepn: Shelton, Van Campen, US 2408345 (1946 to Wm. S. Merrell); Abell et al., US 2590079 (1952 to Wyeth).
Properties: Oily liquid. bp750 205°; bp21 100°.
Boiling point: bp750 205°; bp21 100°
roger2003
(Hive Bee)
08-14-03 15:40
No 453522
... to have instant access to these mentioned patents, which might interest some people.
So I'll add them here:
Shelton, Van Campen, Patent US2408345 (1946 to Wm. S. Merrell)
Abell et al., Patent US2590079 (1952 to Wyeth).
A Dream Within A Dream (http://www.poedecoder.com/Qrisse/works/
(Stunning)
08-14-03 17:26
No 453532
...have no time to translate the post of Fomalhaut about the "simpliest synthesis" :) may bee some other russian bee will do it instead of me ...
Check out: Post 441428 (Fomalhaut: "Фентермин - самый простой стимулятор.", Russian HyperLab)
Good luck!
AllAccess
(Hive Bee)
08-15-03 02:48
No 453616
Phentermine from benzaldehyde and 2-nitropropane
JOC 37, 1861 (1972)
(Chief Bee)
08-15-03 03:12
No 453619
(Rated as: excellent)
Reductive synthesis of
 ,
, -dimethylphenethylamine
-dimethylphenethylamineFritz H. Marquardt, Susan Edwards
J. Org. Chem. 37(11), 1861-1863 (1972) (../rhodium/pdf /phentermine.
(Hive Bee)
02-04-04 23:57
No 486417
why is it neccasary to proceed via the nitro-alcohol?
it would seem to me that a simpler procedure would be to condense the nitronate ion onto a benzylhalide.
while there could be some reaction between the benzylhalide and the base, it would seem that this condition could be managed by combing the 1equiv of nitropropane, 1 equiv of KOH in the ethanol, allow the mixture to sit long enough for deprotonation to occur, and then slowly add in 1 equiv of benzylhalide.
to insure that all the presumably more expensive, 2-nitroprpane is consumed, one could then add for example 0.1equivs of KOH to the ethanol, and slowly drip in another 0.1 equivs of benzylhalide.
since the nitro group is located on a tert carbon, it would seem there would be little danger of the nitro-phentermine undergoing any side reactions.
(Chief Bee)
02-05-04 01:39
No 486447
This may be a side reaction:
Post 204795 (obituary: "Arom. ald. synth", Novel Discourse)
Post 292292 (lugh: "Halides to Carbonyls", Chemistry Discourse)
The Hive - Clandestine Chemists Without Borders
(Hive Bee)
02-06-04 20:07
No 486926
that last article is interesting, it seems the oxidation occurs via the formation of the nitro-ester, followed by its disportiation.
certainly using low temps would greatly slow the disporportation of the nitro-ester, but that would only help if the halide-->nitro-ester reaction is reversible since the alkalytion isn't
(i.e. the equilibrium constant for alkyl-halide + proponitrone<-->alkyl-proponitrone
swim shall put this on his list of experiments worth doing.