12-01-03 10:05
No 474032
Hi Guys,
To reduce the 3,4,5-trimethoxy-nitrostyrene we use LAH in THF or Ehtyl Ether Anydrous. Correct?!? I have a THF with 0,05% of water. Is this amount of water could bring me any problem? In positive case what is the secure water content in THF or Ethyl Ether?
Regards,
Look at the sky... Look at the river... Isnīt it good?
(Chief Bee)
12-01-03 10:20
No 474034
That will work.
(Stranger)
12-01-03 17:22
No 474091
Thank you for answer. I was afraid this amount of water could react with LAH and cause a explosion or to damage my reaction.
Look at the sky... Look at the river... Isnīt it good?
(Chief Bee)
12-01-03 21:34
No 474123
The water will of course react with your LAH, but as you are probably using an excess of LAH anyway, the small amount decomposed will probably make no difference.
(Hive Bee)
12-02-03 16:43
No 474294
Add Natrium or Kalium to your solvent and distill it.
Add a mg Chinhydron an if u can see a color u can be sure no water is present. Always work under inertgas.
(Hive Bee)
12-02-03 19:26
No 474324
inert gas isnt needed, and trace amounts of water donīt disturbe LAH reductions! (because, as rhodium has said an excess of reagent is always used)
...grignard reactions are another topic...
(Moderator)
12-05-03 13:06
No 474789
(Rated as: good read)
Try to calculate the moles of water present in your solvent. You'll be surprised how much it actually is due to the extremely low molecular weight of water.
Buy molecular sieves and store your pre-dried solvents over them. It can be regenerated in a vacuum-oven. And remember that you have to keep (non-stabilized) ether and THF over NaOH against dangerous ether peroxides.
(Hive Bee)
12-05-03 16:57
No 474824
(Rated as: excellent)
Recently read at the ......
.
http://www.orglist.net Everybody digest, Vol 1 #307 -
"1. How to treat wet THF to bring the ppm levels of water down to say, 100 ppm.
2. What is the best way for making super-dry THF.
Q-1:
This is a much discussed question as to how to dry wet THF.
The age old method, that is still being used extensively in the third world countries is to shake THF with KOH flakes. (THIS IS A HAZARDOUS METHOD, AND BANNED THROUGH OUT WESTERN WORLD).
Alternatively, you can dry wet THF over Molecular Sieves, 4A type, properly activated.
(An excellent method for activating molecular sieves is to place them in a microwave oven (kitchen type, full power, 5 minutes)}.
TYPICALLY, IN INDIAN LABORATORIES, WE PASS THF THROUGH AN ACTIVATED ALLUMINA COLUMN. This not only gives the necessary preliminary drying of THF, but also eliminates the peroxides present if any. The alumina thus treated with THF must be considered as A PEROXIDE ITSELF, AND MUST BE DISPOSED OFF ACCORDINGLY. (the organic peroxides BIND to the Alumina strongly).
At an Industrial level, if you have a continuous necessity for distilling THF, you might employ an Alumina column. For one time use, it is NOT viable because, lot of THF will be held in the column (which is obviously a waste). Then, when to replace Alumina in the column? (when you get a Positive peroxide test for you THF and/or when the Moisture content exceeds that of the average five batches of THF you obtained from the same column. [ typically, for 5 Ltrs of THF we used about ONE kilo of Alumina. We never tried to re-use it, but I am sure, you can treat about 30 Ltrs of THF with ONE kilo of Alumina). This Alumina is then collected in Plastic cans and wetted with water and this is treated as Explosive/Hazardous/Flamable waste.
Q-2.
Lithium Aluminium Hydride is no longer a recommended drying agent for THF, inspite of its uncanny ability to remove peroxides. Best way is to treat the preveously dried THF with Sodium, reflux, cool, add Benzoquinone, reflux, await for the dark blue color to persist atleast for six hours, and then collect the liquid that boils at a constant temperature. In this case, you will not be accumulating huge Quantities of Sodium in your reactor vessel. Be abundantly sure that you do NOT have any leaks in your cooling system. Alternatively, if you have a FLAME and SPARK proof surrounding, go for chilled etheral solvents (dibutylether for example) for circulation in the condesor.
THE REASON WHY YOU MUST TREAT THE THF IS TO ENSURE THAT YOU DO NOT ACCUMULATE UNWANTED SODIUM IN HUGE QUANTITIES IN YOUR REACTION VESSEL.
I am sure you are experienced in treating multi kilogram quantities of sodium accumulated in reaction vessel. In some places it is recovered and converted into sodium sand or NaHMDS."
We're all in this world together,
http://www.aztlan.net
(Newbee)
05-12-04 19:04
No 506677
i kind of like the idea of using anhydrous copper sulfate (white) to dry ether. if water is present copper sulfate hydrate (blue) will be formed, so compound is both dessicator and indicator. i have heard rumor that iron or copper metal can prevent the formation of peroxides over long periods of storage.
(Hive Bee)
05-13-04 22:37
No 506944
This is a discription ho to yield anhydrous ethers.
Look here :
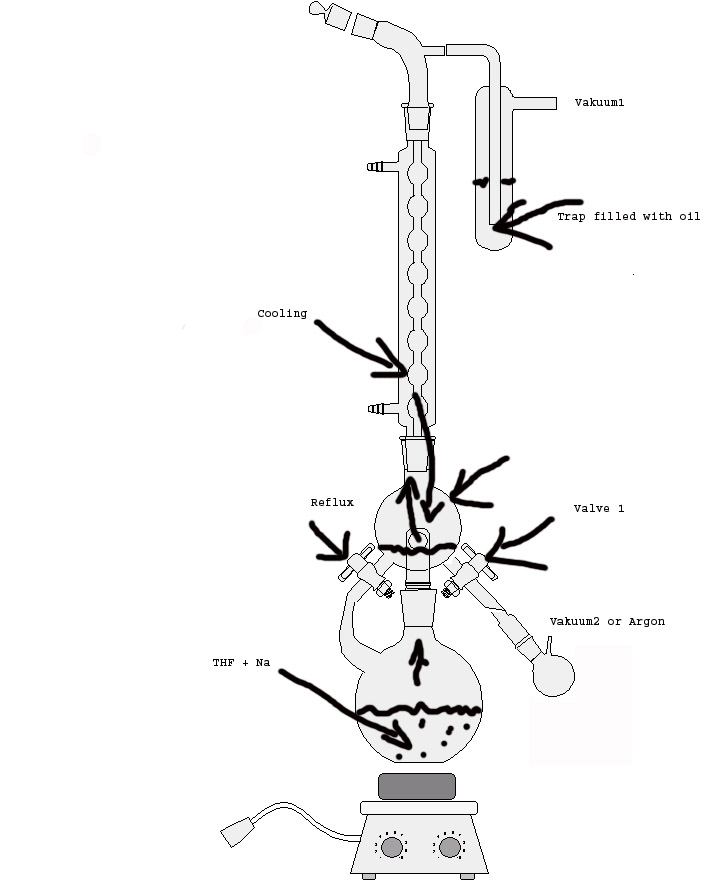
1. evakuate the reaktion apparature through vakuum1.
2. add Ar in the apparatur via valve1 and add THF and Na into the lower vessel.
3.Add a bit Benzophenone to the lower vessel.
4. Reflux till u see a blue color in the lower vessel .
5. Close the left valve and wait until enough THF is in the upper vessel.
6. valve 1 is closed and evakuated via vakuum2.
7. if enogh solvent is present in the upper vessel vakuum2 is stopped and valve1 is opend so that freshly destilled solvent is let to the flask .
8. gas Ar through the flask with the freshly destilled THF.
9. Ready. U have THF with ~10ppm Water