(Hive Addict)
05-08-04 12:46
No 505771
(Rated as: excellent)
THE ALKYLATION OF MERCAPTANS BY MEANS OF SULFONIC ESTERS
By HENRY GILMAN AND NATHANIEL J. BEAKER
In connection with studies involving the interaction of organic sulfur compounds and organomagnesium halides it was necessary to prepare a variety of sulfides. When the hydrogen of a mercapto (-SH) group is to be replaced by alkyl, two classes of compounds are generally used: alkyl halides and salts of alkyl sulfates. Sulfonic esters have been used to a decidedly limited extent in a replacement reaction of this type, despite the fact that these esters find a wide application in the preparation of ethers from the analogous hydroxyl compounds. Dimethyl sulfate has been used in the preparation of methyl sulfides.1 The inherent advantages of cost, smoothness of reaction and high yield of pure product justify the use of dimethyl sulfate as the best reagent for replacing the mercapto hydrogen by methyl. However, the recent availability of diethyl sulfate and a variety of alkyl esters of p-toluenesulfonic acid2 should extend the application of sulfonic esters in the alkylation of mercaptans. It is shown that the yields of sulfides prepared by means of alkyl p-toluenesulfonates are at least equal to those obtained from dialkylsulfates. In most preparations, however, the latter are to be preferred because of the present significant advantage in cost when one considers the alkylating content of the respective esters.
Furthermore, the disadvantages of the costly and volatile alkyl halides disappear in large part when alkyl groups of high molecular weight are to be introduced.
Halogen-alkyl p-toluenesulfonates are available3 However, it is shown that these esters are deserving of very little consideration in the preparation of halogen-alkyl sulfides. It is possible to prepare such sulfides by better methods.4
Experimental Part
A small excess of ester was added to the sodium hydroxide solution of mercaptan. Generally, about a 20% excess of a 15-25% solution of sodium hydroxide was used. An excess of alkali is helpful in breaking down to sulfides5 the sulfonium-sulfides which are formed by the addition of sulfonic esters. With dialkyl sulfates it is desirable to add the ester slowly to avoid the vigorous reaction which often sets in when too much ester is present. The mixture is refluxed for two to three hours after the addition of ester. The oily layer is separated, dissolved in ether, and this solution is washed with water, dried with potassium carbonate and then distilled in a vacuum. In most cases the yield is only slightly decreased by distilling the oily layer directly after separation. The identity of the sulfide was confirmed by converting it to the corresponding sulfone which, in turn, was compared with the known sulfone. Several oxidizing agents have been used for the preparation of sulfones from sulfides. However, it is doubtful whether any of these oxidizers equal in value a 30% solution of hydrogen peroxide. It is necessary only to dissolve the sulfide in glacial acetic acid, add about a 50% excess of the hydrogen peroxide, digest the mixture on a steam plate for a few hours and then pour into water. In this way the sulfone is precipitated in a very high degree of purity and the yield is excellent. In addition to its value as one of the best means of identifying sulfides, it has the merit of being superior to the preparation of sulfones from alkali sulfinates, when the extra cost is secondary to a high yield of pure compound.
(skipped the preparation of ß-Chloro-ethyl p-Tolyl Sulfide)
1 Friedländer, Ann., 351, 390 (1907). Auwers and Arndt, Ber., 42, 537 (1909). Mayer, Ber., 42, 3046 (1909). Zincke and co-workers, Ber., 48, 1242 (1915); Ann., 406, 127 (1914), etc. Pollack and co-workers, Monatsh., 39, 129, 179 (1918). Brand and co-workers, J, prakt, chem., 107,358 (1924), etc. Fricke and Spilker, Ber., 58,24 (1925).
2 Gilman and Beaber, THIS JOURNAL, 47, 518 (1925).
3Clemo and Perkin, J. Chem. Soc., 121,642 (1922). Also Gilman and Beaber, THIS JOURNAL, 45,839 (1923).
4Fromm and Kohn, Ber., 54, 320 (1921).
5 Kehrmann and co-workers, Ber., 55, 2346 (1922), etc. Also Brand and Stall
mann, J. prakt. Chem., 107, 358 (1924).
TABLE I: REACTION OF MERCAPTANS WITH SULFONIC ESTERS
| Mercaptan | G. | Moles | Estera | G. | Moles | Sulfide | G. | Yield % | Boiling point,°Cb |
| n-Butyl | 25 | 0.28 | Ethyl | 60 | 0.3 | Ethyl n-butylc | 18.5 | 78.4 | 143-145 |
| Benzyl | 25 | 0.2 | Ethyl | 44 | 0.22 | Ethyl benzyl | 26 | 85.5 | 220-223 |
| Thiophenol | 22 | 0.2 | Ethyl | 40 | 0.2 | Ethyl phenyl | 18 | 65.2 | 202-205 |
| Thiocresol | 50 | 0.4 | Dimethyl sulfate | 58 | 0.45 | Methyl p-tolyl | 44.5 | 80.6 | 104-105 / 20mm |
| Thiocresol | 100 | 0.8 | Diethyl sulfate | 121 | 0.85 | Ethyl p-tolyle | 95.5 | 83.4 | 101-103 / 2mm |
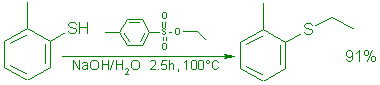
| Thiocresol | 62 | 0.5 | Ethyl | 110 | 0.55 | Ethyl p-tolyl | 69 | 91 | 219-220f |
| Thiocresol | 24.8 | 0.2 | n-Propyl | 47.1 | 0.22 | n-Propyl p-tolylg | 28.5 | 85.8 | 234-235 |
| Thiocresol | 62 | 0.5 | n-Butyl | 125 | 0.51 | n-Butyl p-tolylh | 82 | 91 | 1135-138 / 15 mm. |
a The esters, unless otherwise mentioned, are those of p-toluenesulfonic acid.
b The temperatures recorded in this paper are uncorrected.
cThis sulfide was oxidized to ethyl n-butyl sulfone which is best crystallized from hot petroleum ether: m. p., 50-50.5°.
Anal. Calcd. for C6H1402S: S, 21.33. Found: 21.46.
d Performed by James E. Kirby. All of the methyl p-tolyl sulfide obtained in this experiment was oxidized by hydrogen peroxide. The yield of sulfone was 90%.
eWhen 15-50g. lots of this sulfide were oxidized by nitric acid or acidified potassium permanganate, the yield of sulfone was quite low.
f The ethyl p-tolyl sulfide distilled at 122-125° (20 mm.).
g n-Propyl p-tolyl sulfide boils at 120° (15 mm.); d420 , 0.9755.
hThe time of refluxing with ester in this experiment was 1.5h. At atmospheric pressure, the sulfide distils at 249-250°C with slight decomposition. This distillate on standing deposited a small quantity of fine, white needles which were identified as p-toluenesulfonic acid; d420, 0.9615.
J.Amer.Chem.Soc.; 47; 1925; 1451
--psyloxy--