05-10-04 02:39
No 506098
Does anybody know anything about the activity of the following two compounds?
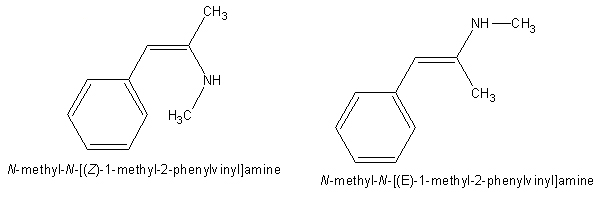
To wit, I am wondering, if I take ephedrine/pseudoephedrine and dehydrate it (somehow; I guess you couldn't just stick it in excess acid...could you?) to give the above two phenylvinylamines, will they be psychoactive? And if so, will the cis isomer be more psychoactive than the trans?
(acetaminophanatic)
05-10-04 03:45
No 506107
Good question.
I guess they'd bee metabolized very quickly.
I think the second one would bee the active one, based on the ring-closed amphetamines I saw. There was MDMA and MDA analogs like that.
I always wondered what would happen if one were to N-ethylynate amphetamine. (i.e. N-ethynyl amphetamine)
...it's an AOL chatroom for dyslexic spider monkies...
(Chief Bee)
05-10-04 08:22
No 506161
Phenylvinylamines aren't stable, as they isomerize to the corresponding imines, and then hydrolyzes.
The Hive - Clandestine Chemists Without Borders
(Stranger)
05-13-04 22:49
No 506947
It would be an interesting question to have answered, if somebody could figure out some way to rotation-restrict the 1,2 ethyl bond.
(Chief Bee)
05-13-04 23:35
No 506955
It has been done, sort of. By using a cyclopropyl group instead of an ethyl chain, it is possible to place the amine in four different positions in respect to the aromatic ring (R-cis-, S-cis-, R-trans- and S-trans-1-phenyl-2-cyclopropylamine).
One article dealing with this is ../rhodium/pdf /nichols/nicho
The Hive - Clandestine Chemists Without Borders