05-20-04 16:16
No 508433
I've been diggin thru the 250+ posts that deal with preparation of Br2. In most of them the end-product is bromine water, or a Br2 soln. in AcOH. There is contradictionary opinions as to wheter bromine vapor can be easily condensed or not. It is known that alcohols and unpolar solvents dissolve Br2 quite well. Freezing out Br2 from, for example, DCM looks like a better way, yet my knowledge is very limited here, so before the respective articles I've ordrerd come in, I want to ask the hives bromine production committee, which would be the most handle-able way to get bromine that is pure enough to use in Post 463377 (psyloxy: "alpha-bromopropanoic acid - a final word ?", Chemistry Discourse) - when amounts of like 1Kg are the goal and Cl2 is to be avoided.
Choose from any of these possible starting materials:
Bromide source: NaBr, KBr, HBr
Oxidizers: H2O2,K2Cr2O7,KMnO4,MnO2,K2S2O8, Na/KClO3
Solvents: AcOH, AcO-R ,DCM, CHCl3, MeOH/EtOH etc., DMF, pet ether/hexane etc., THF, Et2O
--psyloxy--
(Hive Bee)
05-20-04 17:04
No 508440
How about fractionally distilling the bromine after generating bromine from some oxidation method and condensing the vapors using NH4Cl/H2O at -6,5 °C as a coolant system running through a long condenser. The receiving flask should then bee submerged in a cold bath with a temperature of -15 °C or lower.
Hippler
(Hive Addict)
05-20-04 19:22
No 508459
Sounds reasonable enough, but:
a) problems were reported getting the Br2 vapor to condense
b) the ideal procedure should only utilize standard lab-equipment
b) I think a Br2 solution in whatever solvent stores better / is easyer to handle (no need to cool it like mad)
I guess the main questions I have are:
1) which impurities are in the raw Br2 ? ... mainly HBr/H2O I think - how are they removed ?
2) where do I get info on how much bromine can be dissolved in alcohols / DCM / CHCl3 etc...
3) how is bromine recovered from such a solution (without distillation) ? Is freezing out possible ?
4) when catching gaseous Br2 in H2O, after the solution is saturated, where will the Br2 go ? will it form a bottom layer or will it escape as a red cloud again ?
4.5) If it forms a bottom layer, what would be a good way to purify it (I don't think H2O or a little HBr would interfere too much with the propanoic acid bromination, but who knows what HOBr does to it ? )
You see, I'm a complete newbee in that area, plz bear with me!
--psyloxy--
(Hive Bee)
05-20-04 19:37
No 508462
1) which impurities are in the raw Br2 ? ... mainly HBr/H2 I think - how are they removed ?
Fractionating the Br2 should leave no impurities in the bromine afaik.
) where do I get info on how much bromine can be dissolved in alcohols / DCM / CHCl3 etc...
From the Merck index:
Total soly in water (25°): 0.2141 moles/l with formation of 0.00115 moles/l of HOBr; freely sol in alc, ether, CHCl3, CCl4, CS2, concd HCl, aq solns of bromides.
3) how is bromine recovered from such a solution (without distillation) ? Is freezing out possible ?
I dont think that is possible without distillation.
4) when catching gaseous Br2 in H2O, after the solution is saturated, where will the Br2 go ? will it form a bottom layer or will it escape as a red cloud again ?
The gaseous bromine will escape to wherever it can go to
And as for the propanoic acid bromination, perhaps it would bee nice to brominate the propanoic acid "in situ" using for example H-Br/H2O2 ?
Hippler
(Hive Addict)
05-20-04 22:09
No 508479
Fractionating the Br2 should leave no impurities in the bromine afaik.
a) How would that look practically ? Like what length of a coloumn/type of filling would be needed ? Has anyone got an actual written protocol ?
b) I think fractionation would mainly be done to get rid of the azeotrope of water and HBr, boiling at 122°C. If one used an excess of oxidizing agent, there should be no HBr left - right ? Then you could do a normal distillation at 58°C collecting wet bromine.
Which leads to my next thought: extrapolating the facts given in ../rhodium
/tma2.va
separation of Br2 from CHCl3
I dont think that is possible without distillation.
What about this: If you took your bromine saturated chloroform and cooled it to -10°C the bromine (mp -7.2°C) would turn into a solid, the chloroform (mp -63°C)would remain a liquid and could be decanted.
The gaseous bromine will escape to wherever it can go to
Have you heard of 'catching bromine under sulfuric acid' ? Seems like Br2 vapor bubbled into H2SO4 gives a bottom layer of liquid bromine. Purification from there on remains in question.
And as for the propanoic acid bromination, perhaps it would bee nice to brominate the propanoic acid "in situ" using for example H-Br/H2O2 ?
You definately cannot mix propanoic acid, P2O5, H3PO4, H2O2 and HBr to get the bromopropanoic acid, as the water would destroy the polyphosphoric acid, generated from P2O5 and H3PO4, which is the essential ingredient to let the bromination happen at the position next to the -COOH group, not the -CH3 at the end of the chain.
However, maybe you could just bubble Br2 gas into the polyphosphoric acid/propanoic acid mix as it is liberated from whatever bromide oxidation reaction mixture. Maybe minor impurities don't fuck it all up.
Thanks for the answers so far!
--psyloxy--
(Heavyweight Chempion(eer))
05-21-04 01:07
No 508494
I would use a three-neck round-bottom flask equipped with a pressure-equilized addition funnel for the generation of bromine from a concentrated aq. HBr solution by the addition of 30% H2O2. One neck is for the addition funnel, one for the thermometer and the third is used to connect the reaction vessel with a cooling/collecting device using a short liebig condensor.
The cooling/collecting device should be a cold-finger condensor charged with dry ice/DCM*. Below the cooling device is a collecting flask submerged in a cooling bath in which the temp is kept a bit below the freezing poing of bromine.
So, the generation of bromine is done using conc. HBr/H2O2 while keeping the temp above the boiling point of bromine. Then leading the vapour straight away to the condensing/collecting device where the bromine is freezed. I don't think I need to mention that all parts should be made of ground glass?..
* The cooling mix of DCM/dry ice is my own choice since it is not flammable and can't generate a violent lachrymator like bromoacetone as a cooling mix of dry ice/acetone can if one fucks up somehow.
Don't store bromine as a solution in some solvent as it reacts with pretty much everything given time. Purify it and store it in a well capped glass flask in a fridge.
Severe Aztecoholic and President of Sooty's fanclub - Sooty for President!!
(Hive Addict)
05-21-04 01:37
No 508500
Barium, H2O2/HBr sounds like a cool clean way. Can you say anything about water ending up in the collected product ? Neglectable amounts ?
Now that I've been thinking about it the whole day, it strikes me that when Br2 reacts with propanoic acid, HBr is liberated anyways so it shouldn't matter if it's already in the bromine (if it gets in there at all). A little water is added to the polyphosphoric acid in the original procedure - use less and utilize wet bromine - reasonable enough.
A dry ice/DCM cold finger is definately too dedicated - I really like the thought of collecting the bromine in chloroform (if, after all, it is possible to liberate it again, by freezing or whatever <__ any thoughts on this ?).
Two more Q's: what yield (based on Br-)can typically be reached in such reactions ? Which method of generating bromine would be the most space efficient (rxn schemes involving 0,5% KMnO4 solutions would, for example, be down at the hit-list here)
--psyloxy--
(Heavyweight Chempion(eer))
05-21-04 02:51
No 508515
The freezed bromine is allowed to melt and is then dried by adding it to a equal volume of concentrated sulfuric acid and shaking the mixture. The bromine is then isolated by simple separation. Any chlorine present can be removed by distillation of a mixture of KBr or NaBr/Br2.
Why would bromine crystallise out of a solution in chloroform? If you mix EtOH and water, will water crystallise out at e.g. -5°C? No it won't, because a mixture of two compounds will not have the same physical characteristics as the individual compounds has. You will have to cool the CHCl3/Br2 solution far below -7°C to separate them.
The yield of bromine is very high if you use pure reagents.
The most space-efficient method is probably the industrial method of passing chlorine into a saturated aq. NaBr solution at 70-80°C and condensing the liberated bromine as I described. Although they do not freeze the bromine as far as I know. I just added that detail to make the process a bit safer to you.
Severe Aztecoholic and President of Sooty's fanclub - Sooty for President!!
(Stoni's sexual toy)
05-21-04 11:08
No 508567
Bromine can be purified by shaking with conc. H2SO4, as Barium said.
Imagine a 2L sep funnel or flask filled with nearly 2kg of H2SO4 and 3kg of Br2. Quite some workout for your arms. Try not to drop it.
BUSH/CHENEY 2004! After all, it ain't my country!
www.american-buddha.com/addict.war.1.htm
(Hive Addict)
05-24-04 06:54
No 509172
Two more Q's: what yield (based on Br-)can typically be reached in such reactions ? Which method of generating bromine would be the most space efficient (rxn schemes involving 0,5% KMnO4 solutions would, for example, be down at the hit-list here)
After my first and last preparation of bromine last week, I have a suggestion for you. I tried the bubbling Cl2 through concentrated NaBr solution. I put the NaBr/H20 into a flask with a claisen, threaded tubing inlet(like for thermos), and a deanstark with a coil reflux condenser. I had my chiller pumping a solution of about -15c. I pumped a shitload of Cl2 through it then heated the mixture to distill out the Br2. I had problmes condensing all of the Br2. I did not lose much Br2 but red vapours did escape past the reflux condenser. I had tube adaptor on top of the reflux condenser and the end was submerged in sodium bicarbonate solution with check valve. After all was completed I got about a 90% yield. The tubing and area above the coiled in condenser were all tinged red. I disassembled the apparatus quickly and carefully stoppering each joint as it was un-kecked placed into a nearby bucket of lye water. It was not as bad as expected, but neutralizing and disposing of the materials was time consuming and required more chems that I would like. I did it once more and found that adding a column before the reflux condenser and not usig such a high heat produced less escaping fumes. No smell was ever detected because of the neutralizing trap.
Now to my suggestion. I would not start from hydrobromic acid unless you can buy it already made. The use of bromide salt and oxidiser to make HBr then another oxidiser to make it Br2 is more expensive than I would like it to be making several stages of wastes(conc h2o2, h2so4, kind of scarce here and not the cheapest in lage quants). I did an experiment after the nasty Cl2 method that was interesting. However, I did not complete it as I already had enough Br2 and did not want to deal with the clean up again so soon. I took some K2S2O8 and dissolved in a minimum ammount of water and than took a rather dilute solution of NaBr and added them together. Instant fuming. I started the distillation and collected a few milliliters before discontinuing. I did not know how exactly to determine how much oxidiser was nessecary for the complete liberation of Br2 so I did not attempt it again. Now considering the cost of persulfate OTC it seems to be the easiest, quickest, and cheapest route to producing large quantities of Br2 with only one flask of waste and some dirty glass. Simply neutralize the waste and glass and your done. Before storage I had distilled it, dried it with h2so4, extracted it with hexanes, and then stored it as that. Storing bromine in water gives rise to hydrobromic acid and something else that I can't remember the name for when exposed to light or heat in the presence of water.
Just my two bits. I would highly reccomend doing it with the K2S2O8 because of simpkicity and cost. Not to mention to ease of obtaining the chems. I'd save that conc H2O2 from someting more important, but hey if you can spare it go for it.
(Heavyweight Chempion(eer))
05-24-04 10:43
No 509190
As I said, either use a very efficient condenser or get some dry ice. Because of the high melting point of bromine people tend to think there is no problem to condense all the bromine. But no Sir! The very high vapour pressure of bromine can make it quite a task.
There is a thought behind my reccomendations ya know.
Severe Aztecoholic and President of Sooty's fanclub - Sooty for President!!
(Hive Addict)
05-24-04 23:00
No 509338
Yep K2S2O8 seems like made for the job, can't wait to have a look at these articles:
Ber.; Vol. 35; ( 1902 ) 2267 - 2269.
Ber.; Vol. 38; ( 1905 ) 747 - 751
Thanks for sharing your experience there sYnThO !
As for an 'effective cooler' - since I don't think dry ice is an option - how's about that:
Simple distillation setup, cooler leading into a 3 way connector, the other two ends being: an intense reflux condensor (snake cooler ? ) and the receiving flask, submerged in an ice / NaCl -20°C (MgCl2 - 30°C / CaCl2. 6H2O -40°C) mix. Should suffice ?
--psyloxy--
(Moderator)
05-25-04 03:41
No 509405
(Rated as: good read)
The classical laboratory preparation of bromine was conducted using a tubular retort feeding a side arm flask cooled with ice water:
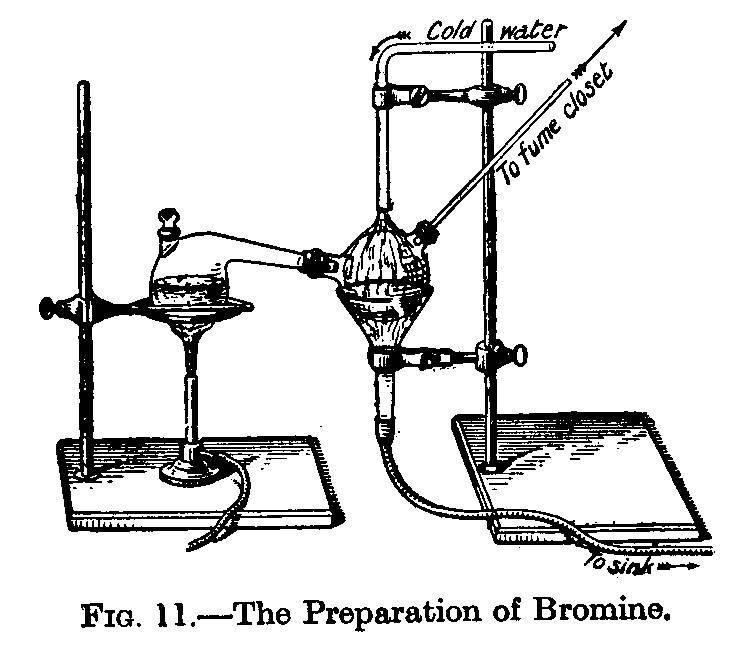
This drawing came from the article on the halogens in Mellor's Comprehensive Treatise on Modern Inorganic Chemistry:

If SWIL was going to attempt producing bromine using standard glassware, a long condenser or two shorter ones connected together fed with ice water would lead into a double necked receiver flask cooled with ice water as shown in the diagram, with the pressure relief on the receiver flask's other neck leading up a reflux condenser cooled with ice water instead of on the connecting tube as is normally done in standard distillation assemblies
Chemistry is our Covalent Bond
(Hive Bee)
05-27-04 07:43
No 509874
Here's an excerpt from SWIM's valuable precursor journal...
OTC Preparation of Pure Liquid Bromine
--------------------------------------
Pure liquid bromine is easy to make OTC with no distillation or chlorine. Make an ice-cold solution of NaBr until no more will dissolve (it's okay if a little remains undissolved). Place the cold solution in an ice-bath and in one portion dump in a large excess of solid oxone and stir rapidly [1]. Liquid bromine will drop to the bottom of the beaker with a little fuming. The bromine is pipetted and dropped into a beaker of concentrated H2SO4 that has been sitting in an ice-salt-water bath. This is stirred well, then the bottom bromine layer is separated and placed in a glass bottle.[2]
[1] If you use 100ml of saturated NaBr solution little heat is generated, at larger scale it is exothermic. Adding some crushed ice to the NaBr solution helps.
[2] Many times the bromine will freeze. Just take the beaker out of the icebath until it melts.