09-23-04 20:06
No 532874
..as read in "the Nitro Group in Organic Synthesis" page 127 the book is found here...
Post 532343 (java: "e-Book: THE NITRO GROUP ORGANIC SYNTHESIS", Chemistry Discourse)
Note: edited by java and corrected by rhodium, should read phenylnitroethanes
It is better to die on your feet than to live on your knees...Emiliano Zapata
(Chief Bee)
09-24-04 00:34
No 532907
Phenylnitropropenes? I can only see a synthesis of Phenylnitroethanes... How is Sulfonylnitromethane best prepared?
The Hive - Clandestine Chemists Without Borders
(Hive Addict)
09-24-04 08:17
No 532949
In my wishful thinking I made an error , sorry about that, as for the arylsulfonylnitromethane synthesis , alI can gather it's a type of Merrifield resin bound nitroacetate and I wasn't able to locate a reference that may have something to do with their preparation.....java
An efficient procedure for the esterification of nitroacetic acid : application for the preparation of Merrifield Bound nitroacetate
J. Org. Chem. 46, 765, 1981
( not able to locate this reference)
It is better to die on your feet than to live on your knees...Emiliano Zapata
(Hive Bee)
09-24-04 23:34
No 533023
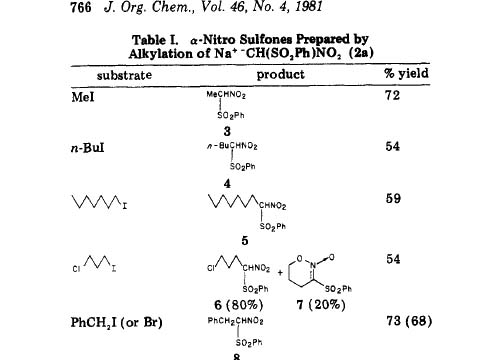
J. Org. Chem. 46, 765, 1981

-SpicyBrown
(Hive Addict)
09-25-04 03:04
No 533041
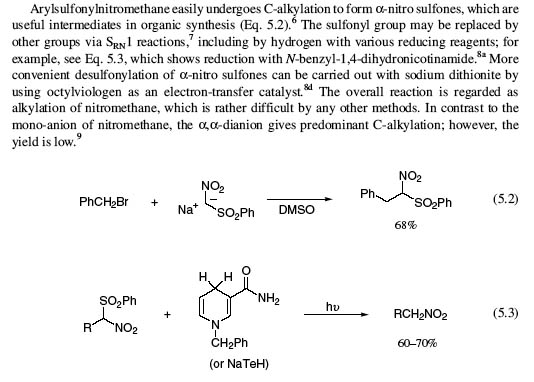
Thank you SpicyBrown for the study , hence the synthesis of the arylsulfonylnitromethane is found and also it seems that nitroethane as well as phenylnitroethane is made and the sulfonyl group is removed as per the original posting ..........java
It is better to die on your feet than to live on your knees...Emiliano Zapata
(Hive Bee)
09-25-04 21:17
No 533153
I feel the need to connect this thread with the analogous one:
Post 475109 (Lego: "Amphetamines/PEAs w/o benzaldehyde or nitroethane", Novel Discourse)
The allkylation mechanism is the same, but removing the –COOMe group is way easier than –SO2Ph. Not to mention that alkyl nitroacetates are way more accessible (even by simply nitrating ethylacetoacetate!).
An interesting method otherwise and potentialy useful for those that have access to such reagents.
“The real drug-problem is that we need more and better drugs.” – J. Ott