(Hive Bee)
07-05-03 12:34
No 444737
(Rated as: excellent)
METABOLISM IN MAN BY CAPILLARY ELECTROPHORESIS
Acta Universitatis Palackianae Olomucensis Facultas Rerum Naturalium 2001 Chemica 40, 25-34 (http://publib.upol.cz/~obd/fulltext/Che
David Jirovsky, b, Juraj Sevcika, b, *, Zuzana Andarovab, Bretislav Smyslc, Petr Bartaka, b, Petr Bednarb, Ales Gavendab and Pavel Adamovskyb
a Laboratory of Bioanalytical Research, Palacky University, tr. Svobody 8, 771 26 Olomouc, Czech Republic
b Department of Analytical Chemistry, Palacky University, tr. Svobody 8, 771 26 Olomouc, Czech Republic
c Institute of Forensic Medicine and Medical Law, Palacky University, Hnevotínska 3, 775 09, Olomouc, Czech Republic
* Corresponding author: +420-(0)68-563 44 16, Fax: +420-(0)68-523 03 56, e-mail: sevcik@aix.upol.cz
Received June 25, 2001
Accepted July 13, 2001
Abstract
Methamphetamine (deoxyephedrine) became excessively abused drug throughout the world. That is the reason why there is an increasing interest in analytical methods for the determination of this drug, especially in biological matrices. The general methamphetamine metabolism in man is known. As methamphetamine has chiral center in the molecule, two optical isomers can be derived. Since metabolic pathways are stereoselective we can expect diverse conversion of individual enantiomers. The study of differences in human metabolism of methamphetamine enantiomers using simple and rapid capillary zone electrophoresis method with cyclodextrin derivative as a chiral agent is reported.
Key words: Chiral separation, Methamphetamine metabolism, Capillary electrophoresis, CZE, methamphetamine, enantiomers, chiral separation, metabolites.
Introduction
Methamphetamine (MAP), a synthetic derivative of ephedrine, is a compound with strong activity on central nervous system. Due to this property it became one of the most abused drugs of today.
It has been established that D-isomer of this optically active amine shows greater physiological effect than the L-form1.
Illicit MAP is available, however, either racemic (and thus optically non-active) or as chiral (+)-D-MAP because there are several different synthetic ways to prepare the drug. The ways of manufacturing also vary from regions and depend on how the starting substance was. In the Czech Republic, for example, abusing of the pure D-form is mainly widespread and the outgoing substance used to be the potent enantiomer of ephedrine, contained in various medicaments easily available.
Because enantiomers differ very often in their effect on living organism, as follows from the asymmetric nature of stereospecific biosystems, there is a significant interest
to study pharmacodynamics and pharmacokinetics of individual drug enantiomers. The main limiting factor for determination and quantification of enantiomers is the lack of sufficiently sensitive and selective analytical methods. Miscellaneous approaches to MAP chiral assay in various matrices are reviewed2. To date, the most frequently used method is HPLC but it does not offer sufficient efficiency for simultaneous baseline separation of several enantiomers of MAP-related compounds3.
Although the general metabolism of MAP in man is well known for several years4, 5, studies of individual MAP enantiomer metabolism are mostly done for animals, however6, 7, or the derivatization to ensure the volatility of the sample prior to GC/MS analysis is needed8. As was mentioned above, diverse metabolism for MAP enantiomers in man can be expected. In case of rats, faster biodegradation of L-form was reported9.
In this paper we describe the application of previously published CZE method with cyclodextrin derivatives as chiral additives10 which proved to be well suited for the investigation of MAP enantiomer metabolism in humans because of its simplicity, low-costs and good enantioselectivity, prior to HPLC3.
Experimental
Chemicals
Optically pure isomers of studied compounds were a gift of FARMAK (Olomouc, Czech Republic) (methamphetamine hydrochloride) and VÚFB (Prague, Czech Republic) (amphetamine hydrochloride, 4-hydroxymethamphetamine hydrochloride, 4-hydroxyamphetamine hydrobromide, norephedrine hydrochloride and 4-hydroxynorephedrine
hydrochloride). The chiral selector hydroxypropyl-beta-cyclodextrin was part of the eCAP(TM) Chiral Methods Development Kit (Beckman Instruments, Fullerton, CA). All other chemicals were of analytical grade and were purchased from Merck (Darmstadt, Germany). The concentration of aqueous solution of studied compounds was 100 µmol/L. 100 mmol/L phosphate buffer was prepared by the titration of
100 mmol/L phosphoric acid using Tris(hydroxymethyl)aminomethane. Deionized water (18 Momega.cm–1) was used for preparation of all solutions.
Urine samples of methamphetamine abusers and healthy volunteers were obtained from Institute of Forensic Medicine and Medical Law (Olomouc, Czech Republic).
Apparatus
CZE experiments were carried out on P/ACE 5510 with diode array detector (Beckman Instruments, Fullerton, CA). All separations were performed in an uncoated fused-silica capillary (50 µm ID, Polymicro Technologies Inc., Phoenix, AZ) at temperature 20 °C. The capillary had an effective length of 40 cm (total length 47 cm). Constant field strength of 200 V.cm–1 was applied. The chiral selector was only present inside the capillary. UV detection was over the range 200–300 nm using a cartridge detection window (100 x 800 µm).
Sample treatment and extraction procedure
Urine samples
Healthy volunteer urine samples were collected 6, 12, 24 and 48 hours after the administration of 20 mg of MAP per oralis. Individual enantiomers (+)- and (–)-MAP and racemate were administered separately.
Enzymatic hydrolysis
To 20 mL urine set up on pH 7.0, 400 µL of beta-glucuronidase/arylphosphatase was added and left to incubate at 37 °C for 24 hours. After this procedure, samples were only diluted in 200 µL of deionised water and further 10 times diluted.
The native urine samples as well as the urine samples treated by enzymatic hydrolysis were extracted as described below:
Extraction
To 20 mL urine was added 1 mL aqueous solution of phenylethylamine hydrochloride (0.158 mg/mL) as an internal standard. Then followed:
– addition of 20% aqueous solution of sodium carbonate, pH set up on 9.5;
– shaking with 40 mL dichloromethane within 5 minutes;
– organic phase shaken with 5 mL water;
– organic phase shaken with 5 mL 0.1M HCl;
– aqueous phase alkalised with 20% aqueous solution of sodium carbonate, pH set up in the range 9.0–10.0;
– shaking with 2 mL dichloromethane within 5 minutes;
– organic phase dried with waterless mercury sulphate;
– addition of 100 µL 0.1M HCl in isopropylalcohol;
– evaporation to dryness in the nitrogen stream.
Results and discussion
For the very first experiments, we chose 100 mM TRIS-phosphate buffer pH = 2.5 as BGE. On the basis of previously obtained results10 with various cyclodextrin (CD) derivatives applied on similar type of compounds, hydroxypropyl-beta-cyclodextrin was
tested. As for other CD’s, it is supposed that non-polar groups of an analyte incorporate into the hydrophobic cavity of CD and the proper enantiorecognition occurs via
polar interactions of the substituents at the mouth of CD molecule. This hydroxypropyl derivative offered successful separation of all potential MAP metabolite enantiomers
except for norephedrine. Increasing the chiral selector concentration up to 100 mmol/L also norephedrine enantiomers exhibit some discrimination, nevertheless,
the achieved resolution RS does not exceed the value 0.44. Also, with the increased concentration of CD deteriorates the recognition between enantiomers of different compounds which makes not possible to separate main metabolites from
each other as well as the analysis time increases.
30 mmol/L hydroxypropyl-beta-cyclodextrin shows to be appropriate concentration allowing to enantioseparate simultaneously all main metabolites of MAP except for
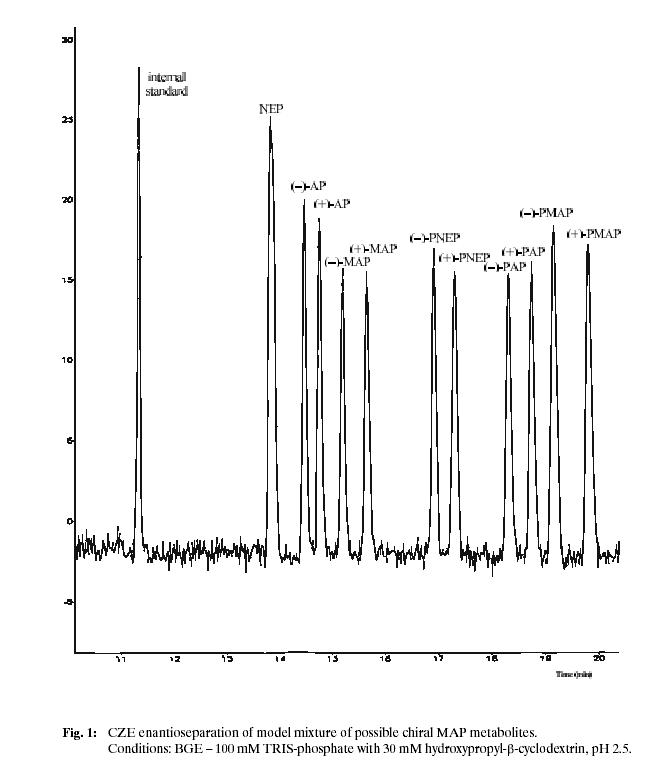
norephedrine within 20 minutes. The achieved separation of model mixture of possible chiral metabolites is shown (see Fig. 1), for experimental conditions see below.
Model mixture containing racemates of amphetamine (AP), MAP, norephedrine (NEP), p-hydroxyamphetamine (PAP), p-hydroxymethamphetamine (PMAP), p-hydroxynorephedrine
(PNEP) and phenylethylamine as an internal standard was used to prepare the calibration curve in the concentration range 1.10–5 up to 5.10–4 mol/L.
The overview of calibration curve equations as well as correlation coefficients is given in the Table 1.
Table 1: Calibration data.
| Compound | Correlation equation | Correlation coefficient |
| phenylethylamine | y = 5.102x + 0.11 | 0.9876 |
| (–)-amphetamine | y = 2.103x + 0.15 | 0.9988 |
| (+)-amphetamine | y = 1.103x + 0.10 | 0.9714 |
| (–)-methamphetamine | y = 7.102x + 0.31 | 0.9721 |
| (+)-methamphetamine | y = 8.102x + 0.19 | 0.9797 |
| norephedrine | y = 1.103x + 0.13 | 0.9567 |
| (–)-p-hydroxynorephedrine | y = 8.102x + 0.41 | 0.9873 |
| (+)-p-hydroxynorephedrine | y = 8.102x + 0.33 | 0.9976 |
| (–)-p-hydroxyamphetamine | y = 8.102x + 0.49 | 0.9666 |
| (+)-p-hydroxyamphetamine | y = 8.102x + 0.57 | 0.9886 |
| (–)-p-hydroxymethamphetamine | y = 1.103x + 0.53 | 0.9972 |
| (+)-p-hydroxymethamphetamine | y = 1.103x + 0.64 | 0.9910 |
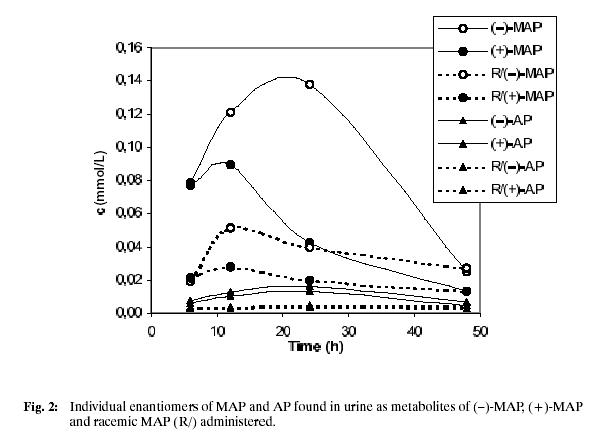
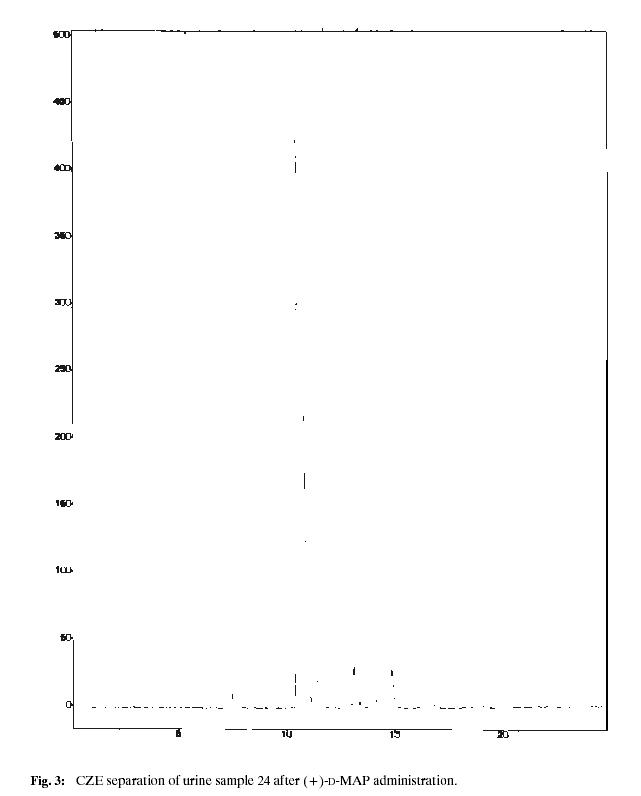
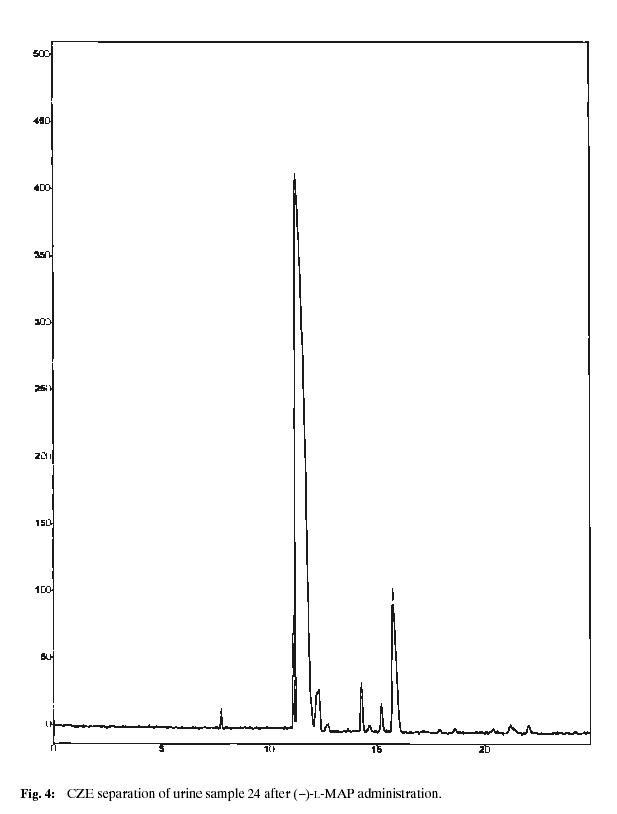
Using calibration curves, amounts of individual metabolites in healthy volunteer urine samples were established. Fig. 2. shows the found results for the each time point of sample collection, Figs. 3 and 4 represent analyses of the urine samples collected after 24 hours after the drug administration. The diversity in individual enantiomer and racemate metabolism is evident, each form of MAP is transformed by a different metabolic pathway. In all cases, (–)-L-MAP, which is physiologically much less active, is excreted mainly in unchanged form, whereas the found concentrations of the opposite (+)-MAP enantiomer are significantly lower. A maximum concentration of MAP in urine can be found in about 12 hours after the oral administration, then its level decreases. It was still possible to detect and separate MAP enantiomers 48 hours after the administration of the drug. For the racemic MAP administered, the situation is very similar, (+)-D-MAP is more distributed and transformed in comparison to (–)-MAP.
Amphetamine, the main metabolite of MAP, was also present in assayed urine samples. Again, the less active (–)-AP isomer was found in higher concentration. This isomer came exclusively from (–)-isomer of MAP, no cross isomerisation was observed.
A maximum amount of AP enantiomers was found in 24 hours after administration of drug. Unlike MAP excretion, the curve profile is flat with slow increase and decrease.
Other MAP metabolites, like hydroxylated forms, we did not observed in urine samples.
The present analytical method is very well suited for the determination of chiral MAP metabolites of man in urine, in comparison to earlier reported HPLC method3
offers higher efficiency and thus better enantiorecognition. A further exact description of the chiral biodegradation of MAP in man is rather more complicated because there are several participating metabolic pathways. A further investigation can contribute
to determine which of them is the most considerable one.
Acknowledgments
The authors thank the Ministry of Education for the support by a grant No. MSM 153100013.
David Jirovský, Juraj Ševèík and Petr Barták gratefully acknowledge the support by a grant of Ministry of Education (No. VS 96021).
References
1. Cho, A. K.: Science, 249, 631 (1990).
2. Jirovsky, D., Lemr, K., Sevcik, J., Smysl, B., Stransky, Z.: Forensic Sci. Int., 96, 61 (1998).
3. Jirovsky, D., Lemr, K., Sevcik, J., Smysl, B., Hlavac, J.: Acta UPOL, 35, 85 (1996).
4. Caldwell, J.: Drug Metab. Rev., 5, 219 (1976).
5. Cook, C. E., Jeffcoat, A. R., Hill, J. M. et al.: Drug Metab. Dispos. Biol. Fate. Chem., 21, 26 (1993).
6. Sukbuntherng, J., Hutchaleelaha, A., Chow, H. H., Mayersohn, M.: J. Anal. Toxicol., 19, 139 (1995).
7. Hutchaleelaha, A., Walters,A., Chow, H. H., Mayersohn, M.: J. Chromatogr. B, 658, 103 (1994).
8. Cody, J. T., Schwarzhoff, R.: J. Anal. Toxicol., 17, 321 (1993).
9. Nagai, T., Kamiyama, S.: J. Chromatogr., 525, 203 (1990).
10. Sevcik, J., Lemr, K., Smysl, B., Jirovsky, D., Hradil, P.: J. Liq. Chromatogr., 21, 2473 (1998).
*EDITED*: Table fixed, thanks Hypo!
http://www.poedecoder.com/Qrisse/works/d
(Hive Addict)
07-05-03 12:53
No 444739
> WTF? What am I doing wrong with my tables here?
second line, last td:
> [td]phenylethylamine[/td] [td]y = 5.102x + 0.11[/td] [td]0.9876[td]
should have a slash
(yeah, table code is kinda picky...)
(Active Asperger Archivist)
07-07-03 06:34
No 445208
damn right it's picky. after about four or five large tables you get used to it though.
Act quickly or not at all.