04-13-04 23:44
No 500741
i've seen them around, in little plastic containers with yellow and green label with red (i can't recall if it was red now, i've not looked at one in a while) at virtually every healthfood store i've ever seen. the only caveat is they're tablets, although i've had some success separating the amino acids from the pills, i'm sure a more precise method could yield much cleaner material. It says on the side of the label 'DL Phenylalanine 500mg' or something like that.
(Hive Martyr)
04-14-04 00:29
No 500745
I knew it was a dumb fucking question but seeing as how I've had these DLPA-750 extra strength capsules, that were bought after a stint in a rehab where they were giving them to patients in for meth abuse and advised me to pick some up to "replace the chemicals in my brain that the meth has depleted from abuse".
Taking all that into consideration, the question was asked.
No offense to the karma fairy for not just looking for the answer myself. I just love it when my chemical hero's answer me in any fashion. Even to tell me to UTFSE.
Rhod's a god in my world. Jade is climbing up the 'keep it real' tree as well.
Enough off topic shit. Just had to explain my insolence.
Smack goes some more bad karma!
(Hive Bee)
07-03-04 17:45
No 517287
Rhodium .... in the procedure using NaBH4 with Iodine to reduce COOH to OH in Phenylalanine........
Post 459750 (Rhodium: "Phenylalaninol, exact procedure", Stimulants)
I have difficulty getting THF and methylene chloride is there any alternative solvents that can be used in this procedure which is quite nice save the solvents needed......java
Just hold on to the thread...that keeps us going
http://www.chiapaslink.ukgateway.net/
(Hive Bee)
07-06-04 18:08
No 517849
i have posted a paper on alkylation of amino acids (protonation of copper glycinate). if a methyl group was added to protonated phenylalanine and this product decarboxylated supposedly amphetamine would be formed. maybe someone could try this and post their results.
(Hive Bee)
07-06-04 18:51
No 517855
Do you mean this post.....
Post 506443 (xxxxx: "protonation of copper glycinate", Novel Discourse)
but you see that takes me in a completely new direction. I will have to look at it see if it has it's merits, thanks for the the tip....java
Just hold on to the thread...that keeps us going
http://www.chiapaslink.ukgateway.net/
09-16-04 19:00
(Rated as: misinforming)
(Stranger)
09-20-04 21:08
No 532435
sorry, i merely wanted to point out that a route exists from phenylalanine to phenylacetaldehyde via bleach to the imine with methylamine and finishing with a methyl grignard, which achieves the meth. Yields? buggers me. Anybee know?
Is the methyl grignard reagent made how i suspect - methiodide plus magnesium turnings? It occurred to me that the stim-bees would be able to make methiodide using a RP/I rxn with methanol. One must have glass for distilling and refluxing when going this route, but that's well worth it anyway.
I think this little scheme would make a great first introduction to all the basic types of processes involved in much of the more interesting syntheses. Oxidation reaction, iodination, grignard, amine depolymerisation, distillation. All fairly basic, a step up from the rp/i and giving some good grounding in the basics. It is unlikely that phenylalanine is going to be off the shelves any time soon so it seems a good small scale method that doesn't require entering a pharmacy to purchase an increasingly adulterated precursor.
edit:
Post 531180 (Kinetic: "Strecker Degradation", Stimulants)
kinetic seems to think that phenylacetaldehyde would be the result of oxidising phenylalanine in the same way as the alphamethyl phenylalanine becomes p2p.
What would be the result of applying the methyl grignard to the phenylalanine - or would it's low solubility make it unworkable? If some way can be found to put on the alpha methyl first one simply decarboxlates for amphetamine or oxidise to p2p and aminate with methylamine for meth - or formylate and reduce the amphetamine. I can't think of any way that would do it but I am only a hobby chemist.
(Stranger)
09-21-04 15:36
No 532577
How about COOH,plus lead acetate?Would that bee usefull??IM no Chemist just a thought.
Big Paper Face Makerô
(Stranger)
09-24-04 02:47
No 532925
Swim did a qualitative test of an oxidation protocol for phenylalanine - 500mg capsule of phenylalanine was placed in a test tube with about 100-150mg of the dichromate and about 15 ml of distilled water. After getting the dichromate to fully dissolve, the first thing that happened was a sweet smell which resembled rose oil, and then as the reaction proceded it finally smelt like marzipan.
When this story was related to me i asked myself what would happen when oxidising it. Is there a point where the reaction would tend to stop at, that is to say, is it possible that the reaction with bleach or permanganate or whatever will tend to result in some decomposed material and mostly benzaldehyde? Not exactly the most cost effective source for it, granted, but if economy is not paramount, still useful.
After watching the reaction SWIM felt that oxidising the phenylalanine would require a very mild oxidiser, i thought perhaps even 6% peroxide would be strong enough. It really doesn't have to be very strongly oxidised at all.
Otherwise, this bleach reaction should be added to the benzaldehyde synths imho, nutrasw**t is a cheap source of phenylalanine and a simple pH separation would yield the material. There is something like 150mg of phenylalanine in an average banana. I believe it is possible to get in powder pure form too.
A further test with 6% H2O2 will be done. The almost rose smelling aldehyde that is formed almost certainly must be phenylacetaldehyde. A gentler oxidiser and a larger volume of water is probably what is needed, there was likely material that remained unreacted.
If the right molar ratio were used, and excess water, would that help increase the ratio of phenylacetaldehyde that is formed?
(Wizard Master)
09-24-04 06:48
No 532946
psi0nic: I commend you at least for experimenting. Let me give you some quick advice.
phenylalanine, C6H5-CH2-CH(-NH2)-COOH will oxidise in the benzylic position. C6H5-CH2-
benzaldehyde, C6H5-CHO will further oxidise to benzoic acid, C6H5-COOH
I rated your earlier post as misinforming for these comments... "The phenylacetaldehyde is easy to make from phenylalanine via oxidation. Magnesium from pencil sharpeners".
phenylacetaldehyde is easily made from styrene via the oxirane or glycol rearrangement.
Styrene Oxide http://www.orgsyn.org/orgsyn/prep.asp?pr
(Stranger)
09-24-04 08:54
No 532953
well a more careful reaction needs to be done because there was definitely a different odour at the beginning than how it ended up. Would it help to have something in there which will extract the aldehydes before they further oxidise as per the strecker document on rhodium?
During the process of breakdown, which things are attacked and in what order? Does the oxidiser attack the NH2 before it starts on the hydrocarbon chain? Seems reasonable to me at least. The decarboxylation would also occur but more so with a more vigourous reaction - this is why i thought of using a very mild peroxide to keep the process slow. If one can simply de-aminate the phenylalanine, would this make it possible to progress to p2p easier? just exploring the logical pathways (but not neccessarily well informed
Another thought - what about decarboxylating the Phe first up to phenethylamine and make some use of this terminal amine as a route to the final product, maybe via the imine.
(Chief Bee)
09-24-04 14:23
No 532982
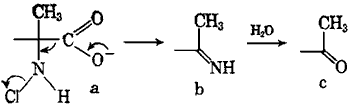
The strecker degradation of amino acids starts with N-chlorination by the hypochlorite ion:
R-NH2 + OCl- -> R-NH-Cl (a, see above)
The N-chloroamine a then decomposes into a chloride ion (Cl-), carbon dioxide and an imine b, the latter next being hydrolyzed to the corresponding carbonyl compound c. Depicted above is the formation of a methyl ketone from an alpha-methyl-aminoacid, but naturally phenylacetaldehyde would be formed from phenylalanine.
The Hive - Clandestine Chemists Without Borders
(Wizard Master)
09-25-04 03:42
No 533044
(Rated as: good read)
http://themerckindex.cambridgesoft.com/T
Interaction of an a-amino acid with a carbonyl compound in aqueous solution or suspension to give carbon dioxide and an aldehyde or ketone containing one less carbon atom. Inorganic oxidizing agents can also be used to bring about the reaction:
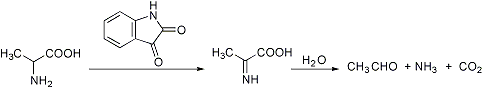
And... The strecker degradation of amino acids starts with N-chlorination by the hypochlorite ion:
R-NH2 + OCl- => R-NH-Cl => imine => aldehyde.
It is very important to get the ratio's of amino acid to hypochlorite correct. The phenylacetaldehyde will further oxidise to benzaldehyde or benzoic acid.
Powerful oxidising agents like KMNO4 will react via the strecker degradation of phenylalanine, BUT also attack the benzylic -CH2- on the phenylalanine.
Example. phenylalanine =[KMNO4]=> C6H5CHO + H2C=O + NH2 + CO2
A. Oxidation of Alkyl Side-Chains http://www.cem.msu.edu/~reusch/VirtualTe
The benzylic hydrogens of alkyl substituents on a benzene ring are activated toward free radical attack, as noted earlier. Furthermore, SN1, SN2 and E1 reactions of benzylic halides, show enhanced reactivity, due to the adjacent aromatic ring. The possibility that these observations reflect a general benzylic activation is supported by the susceptability of alkyl side-chains to oxidative degradation, as shown in the following examples (the oxidized side chain is colored). Such oxidations are normally effected by hot acidic pemanganate solutions, but for large scale industrial operations catalysed air-oxidations are preferred.
Bond Dissociation Energy and Radical Stability
http://www.cem.msu.edu/~reusch/VirtualTe
(Hive Addict)
09-29-04 02:53
No 533672
"In any case, once upon a time that was the worst of the adulterants to cold pills so what the hey - certainly seem to me to be totally facile to ethanol."
I appreciate the info but after further investigation, at 1mg per ml of ethanol one could get a whopping 1g yield from the capsule sediment for every 1 liter jar of alkie. Not exactly cost-effective. 90 liters per 90 gs?
Swim found another source containing L-PAA and mag stearate. Mag stearate is insoluble in water. At 50mg L-PAA per ml in dh20, he will bee waiting awhile for some serious evaporation.
Evaporating 5 liters of dh20 is better then 90 liters of denatured but on 2nd thought, does anyBEE know if magnesium stearate would even interfere with an electrolytic PAA oxidation to amphetamine?
Refuse/Resist
(Stranger)
09-29-04 07:32
No 533722
ah ok i see now, might help if it were to actually *read* the thing before thinking about it *slaps head* (let alone post about it)
It specifically says, dissolve amino acid in water, add to nonpolar solvent, then add the bleach solution *very* slowly and use iodine stained starch to determine the completion of the chlorination, which is the main step that makes the reaction work. it is a delicate reaction obviously enough. The instructions should pretty much apply identically as phenylalanine except for the need to change the molar weight of substrate to match the amount of hypochlorite.