02-03-04 23:36
No 486303
I am interested (for academic reasons, of course) in the conversion of styrene to phenylacetic acid, thence to P2P through distillation with Ca(OAc)2
I've seen the Willgerodt reaction from styrene oxide to PhAcOH, but it produces H2S, clearly something to be avoided. Most of the patents I've seen for the creation of PhAcOH from styrene oxide involve high temperatures, in excess of 200C.
My question is: will the performic oxidation of isosafrole, as detailed in ../rhodium /peracid
Yields are not a huge issue, as along as the reagents are cheap, and the finished product is easily separated.
Thanks in advance,
MethyMouse
P.S. To head off some responses which are sure to follow: Yes, I am well aware of the various synths of phenylacetic acid on Rhodium's site. No SWIM will not buy phenylacetic acid or phenylethanol or phenylethane. SWIM does not want to perform a Grignard synthesis.
I've got the kind of toys you've never seen
Manmade and a bit obscene
(Stoni's sexual toy)
02-04-04 01:05
No 486310
You can do the alcohol Wacker with styrene. Depending on what catalyst you use you will either end up with acetophenone or phenylacetaldehyde. I think PdCl2/CuCl2 will yield the former and Pd(NO3)2/Cu(NO3)2 will yield the latter. Could be the other way round too, don't remember for sure.
I'm not fat just horizontally disproportionate.
http://tinyurl.com/3kdu
(Wizard Master)
02-04-04 05:56
No 486328
PHENYLGLYOXAL
http://www.orgsyn.org/orgsyn/prep.asp?pr
PHENYLACETAMIDE
http://www.orgsyn.org/orgsyn/prep.asp?pr
(Hive Bee)
02-04-04 09:09
No 486340
See
Patent US3452047
and
High-yield synthesis from styrene oxide or styrene glycol: G. Paparatto, G. Gregorio, Tetrahedron Letters 29, 1471 (1988).
.
(Hive Bee)
02-04-04 12:13
No 486372
What? P2P from Styrene?????
Is that like poly styrene?
Does that mean I can smash up my Eski and make speed?
Wow Cool!
J/K
(Hive Bee)
02-04-04 13:16
No 486389
im sure some interesting products can be made from polystyrene and ive actually been doing some research on it , but i believe methymouse is talking about styrene monomer.
a given reaction on polystyrene would most likley give a very different product then the same reaction on the monomer.
about the only thing ive figured out to make from polystyrene thus far is phenol, (by HBr catayzed air oxidation). this to my knowledge is not been done experimentally, but similiar reactions with cumene would indicate that this would be a likley result.
and back to your post methymouse:
what about diol formation (e.g with permangenate), followed by dehydration with H2SO4 to yield phenylacetylaldehyde, followed by oxidation to phenylaceticacid?
(Hive Bee)
02-04-04 13:42
No 486398
In a simple step you can convert polystyrene to styrene
Patent US2372528
roger2003
(Hive Bee)
02-04-04 14:29
No 486409
"What? P2P from Styrene?????
Is that like poly styrene?"
Of course you need the monomer, but that's not hard.
SWIM bought two packages of polystyrene cups (not the styrofoam kind, the thin clear ones), cut them up, and put them into a flask. He then heated the flask until styrene started to distill over. This was not done under inert gas, and product was somewhat yellow (like urine). Redistillation yielded ~200ml styrene, clear to very light yellow.
The only real difficulty was the rock hard residue at the bottom of the flask. This was refluxed with a KMnO4 solution until it could be broken apart.
Not hard. The hard part is getting anything useful from it.
I've got the kind of toys you've never seen
Manmade and a bit obscene
(Hive Bee)
02-04-04 14:39
No 486411
Ah! I hadn't thought of using KMnO4, which definitely should work. The performic reaction would be nicer (no obnoxious MnO2 preciptate if the reaction is basic) and use gentler chemicals. Is there any reason why it shouldn't work?
Would KMnO4 + H2SO4 + Styrene (cold) yield PhAcOH directly, or would SWIM have to isolate the intermediate stages?
Roger: SWIM definitely tries to avoid using thallium salts! I'll check out the Tetrahedron reference next time I'm at the chem. library. Thanks!
I've got the kind of toys you've never seen
Manmade and a bit obscene
02-04-04 23:54
(Rated as: insignificant)
(Hive Bee)
02-05-04 06:58
No 486585
It's rather unclear to me how exactly this is excellent. I haven't done anything other than distilling polystyrene cups and posting theoretical syntheses which may or may not work. SWIM should be able to try the KMnO4 thing next week (though without the H2SO4--I remembered that acids polymerize styrene).
I've got the kind of toys you've never seen
Manmade and a bit obscene
(Hive Bee)
02-05-04 15:07
No 486686
regarding the rock hard resin left after distilling your cups, some polystyrene has some p-pheynl-diethene in the mix to create cross links and a stiffer plastic, i wonder if using a crappier grade of polystyrene would reduce the amount of resin left.
sounds like it might be worthwhile to jury rig a condensor to a disposable vessel next time, like an empty paint can or something.
Would KMnO4 + H2SO4 + Styrene (cold) yield PhAcOH directly, or would SWIM have to isolate the intermediate stages?
in order to not get clevage(and i don't mean the good kind of cleavage
but as to what youd need to do from there id guess id recommend looking at the article in tet. letters mentioned by rodger.
(Hive Bee)
02-06-04 01:13
No 486861
See:
Post 322863 (Elementary: "Styrene oxide -> Phenylacetaldehyde", Chemistry Discourse)
roger2003
(Stranger)
02-09-04 19:29
No 487589
See
Post 258134 (foxy2: "New preparation of phenylacetic acid", Chemistry Discourse)
(Hive Bee)
02-10-04 19:18
No 487796
(Rated as: good read)
"High-yield synthesis from styrene oxide or styrene glycol: G. Paparatto, G. Gregorio, Tetrahedron Letters 29, 1471 (1988)."
Unfortunately, this is a gas-phase reaction at ~200C. However, while looking this up at the library, I found the following other references:
Oxidation of styrene derivatives by peroxydisulfate(2-) ion-copper(II) in acetic acid and acetonitrile. Reaction paths in oxidations via radical cations
Cheves Walling, Gamil M. El-Taliawi, Kalyani Amarnath;
J. Am. Chem. Soc.; 1984 ;106 (24); 7573-7578.
Notes: This paper gives a method for oxidizing styrene to phenylacetaldehyde and benzaldehyde (yield ratios vary depending on the condentration). Everything looks pretty nice, especially if your goal is benzadehyde. The real question is whether this method can be applied to something like anisole...
The Preparation of Cinnamaldehydes by the Formylation of Styrenes
Claude J. Schmidle, Patricia G. Barnett;
J. Am. Chem. Soc.; 1956 ;78 (13); 3209-3210.
Notes: Styrene+phosphorus oxychloride+DMF-->cinnamaldehyde.
The Reaction of Styrene Oxide with Methylmagnesium Iodide
Calvin Golumbic, D. L. Cottle;
J. Am. Chem. Soc.; 1939 ;61 (5); 996-1000.
Notes: Grignard addition of MeI to styrene oxide gives P2Pol in 53% yield.
Simple Iron Catalyst for Terminal Alkene Epoxidation
Dubois, G.; Murphy, A.; Stack, T. D. P.;
Org. Lett.; (Communication); 2003 ;5(14); 2469-2472.
Abstract: A mu-oxo-iron(III) dimer, [((phen) 2(H 2O)Fe III )2(mu-O)](ClO 4)4, is an efficient epoxidation catalyst for a wide range of alkenes, including terminal alkenes, using peracetic acid as the oxidant. Low catalyst loadings, in situ catalyst preparation from common reagents, fast reaction times (<5 min at 0 C), and enhanced reaction performance at high substrate concentrations combine to create a temporally and synthetically efficient procedure for alkene epoxidation.
Synthesis of Phenethyl Alcohol by Catalytic Hydrogenation of Styrene Oxide
Yadav, V. G.; Chandalia, S. B.;
Org. Process Res. Dev.; (Article); 1998 ;2(5); 294-297.
Abstract: In the current work, phenethyl alcohol was synthesized by the hydrogenation of styrene oxide, which may be obtained by the oxidation of styrene. Conventional method of synthesis of phenethyl alcohol is by the Friedel-Crafts alkylation of benzene by using ethylene oxide and molar quantities of aluminum chloride. This method causes effluent disposal problems due to the stoichiometric use of aluminum chloride as the aluminum oxychloride formed in the process is normally not recycled. The hydrogenation of styrene oxide was conducted by using Raney nickel catalyst and process conditions were established. The yield of phenethyl alcohol obtained was 90.2% with 92% overall conversion of styrene oxide under suitable conditions. Styrene oxide was also synthesized in the laboratory.
I've got the kind of toys you've never seen
Manmade and a bit obscene
02-10-04 22:59
(Rated as: insignificant)
(Chief Bee)
02-11-04 05:47
No 487882
(Rated as: excellent)
Oxidation of styrene derivatives by peroxydisulfate(2-) ion-copper(II) in acetic acid and acetonitrile.
Cheves Walling, Gamil M. El-Taliawi, Kalyani Amarnath
J. Am. Chem. Soc. 106, 7573-7578 (1984) (../rhodium/pdf /styrene-ox.p
Abstract
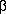 -Aryl carbonyl compounds are major products in the oxidation of a variety of styrene derivatives by S2O82- Evidence is presented that they arise via oxidation to a radical cation, nucleophilic addition of water to give a
-Aryl carbonyl compounds are major products in the oxidation of a variety of styrene derivatives by S2O82- Evidence is presented that they arise via oxidation to a radical cation, nucleophilic addition of water to give a 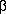 -hydroxyalkyl radical, CuII oxidation to epoxide, and finally acid-catalyzed rearrangement. Data on oxidation of alkyl aromatics with additional functional groups are presented. With ether and amino groups, oxidation occurs at the functional group even when it is remote from the aryl nucleus. These and previous data are summarized to give a coherent picture of the various paths by which aryl side chains may be degraded via initial radical cation intermediates.
-hydroxyalkyl radical, CuII oxidation to epoxide, and finally acid-catalyzed rearrangement. Data on oxidation of alkyl aromatics with additional functional groups are presented. With ether and amino groups, oxidation occurs at the functional group even when it is remote from the aryl nucleus. These and previous data are summarized to give a coherent picture of the various paths by which aryl side chains may be degraded via initial radical cation intermediates.The article has been discussed briefly in Post 122760 (dormouse: "JACS,106,24,(1984) 7573-7578; ammonium persulfate CuII complex as an oxidizer", Serious Chemistry)
____ ___ __ _
The Reaction of Styrene Oxide with Methylmagnesium Iodide
Calvin Golumbic, D. L. Cottle
J. Am. Chem. Soc. 61, 996-1000 (1939) (../rhodium/pdf /styrene.oxid
Summary: Grignard addition of MeI to styrene oxide gives P2Pol in 53% yield.
The article has been posted before in Post 415832 (Chimimanie: "First ref", Novel Discourse)
The Hive - Clandestine Chemists Without Borders
(Wonderful Personality)
02-11-04 15:00
No 487963
"High-yield synthesis from styrene oxide or styrene glycol: G. Paparatto, G. Gregorio, Tetrahedron Letters 29, 1471 (1988)."
Unfortunately, this is a gas-phase reaction at ~200C.
If somebody could look this up please?
For the case that there is no odd catalyst needed this sounds like a winner to me....
....connect the outlet of the tube by a nozzle with the inlet of another tube loaded with MgO - a second nozzle adding GAA - and collect the formed P2P ?
Sounds to good to be true!
(Chief Bee)
02-11-04 15:12
No 487968
Tetrahedron Letters, Volume 29, Issue 12, 1471-1472 (1988)
DOI:10.1016/S0040-4039(00)80328-7
Abstract
Phenylacetaldehyde is synthesized in high yield by isomerization of styrene oxide and by dehydratation of styrene glycol in gas phase using H-ZSM-5 zeolite, Silicalite and silica gels as catalysts.
The Hive - Clandestine Chemists Without Borders
(Hive Bee)
02-11-04 17:23
No 487995
"....connect the outlet of the tube by a nozzle with the inlet of another tube loaded with MgO - a second nozzle adding GAA - and collect the formed P2P ?"
I'm highly skeptical that this would work. This synthesis forms phenylacetaldehyde, not phenylacetic acid. That's a big difference, since the P2P is formed by by addition of an Ac group to the toluene radical formed by the decarboxylation of the PhAcOH.
Here's two more references. The first is perhaps mildly interesting, especially if Pd/C could be replaced with with Zn. The second is a wonderful review article of reactions of substituted and unsubstituted styrenes, including a nice review of reduction methods for substituted beta-nitrostyrenes. It also indicates that if the permanganate diol synthesis is to be done (and based on a book I looked at on permanganate oxidation, it can), it must be kept very cold. Suggested cosolvents in the book were ethanol+MgSO4 or acetone with together with slow addition of AcOH as the reaction progresses, keeping a pH of 9.
Palladium Catalyzed, Regioselective Reduction of 1,2-Epoxides by Ammonium Formate
Peter S. Dragovich, Thomas J. Prins, Ru Zhou;
J. Org. Chem.; 1995; 60(15); 4922-4924.
The Reactions of Monomeric Styrenes.
William S. Everson;
Chem. Rev.; 1949 ;45 (2); 183-345.
I've got the kind of toys you've never seen
Manmade and a bit obscene
(Chief Bee)
02-11-04 17:37
No 488000
(Rated as: good read)
Palladium Catalyzed, Regioselective Reduction of 1,2-Epoxides by Ammonium Formate
Peter S. Dragovich, Thomas J. Prins, Ru Zhou
J. Org. Chem. 60, 4922-4924 (1995) (../rhodium/pdf /cth.epoxides
____ ___ __ _
The Reactions of Monomeric Styrenes
William S. Everson
Chem. Rev. 45, 183-345 (1949) (../rhodium/pdf /styrene.rxn.
The Hive - Clandestine Chemists Without Borders
(Hive Bee)
02-14-04 13:06
No 488656
There is apparently an enormous body of literature concerning the conversion of acetylene to acetaldehyde, notably the use of a mercury(II) salt, which reacts with the acetylene, and is then hydrolized to acetaldehyde. I wonder of the same technique could be applied to the manufacture of phenylacetaldehyde from styrene. Patent Patent GB312716 is especially interesting, since it lists the possibility of using sodium bisulfate and a Cu(II) salt--forgoing the mercury. See Post 435662 (lugh: "From Acetylene", Novel Discourse)
Styrocrank may yet be a reality.
So far the most promising routes seem to be the reduction of styrene epoxide to beta-phenethyl alcohol with Pd/C and NH4COO, the Pinacol rearrangement of the 1,2-diol with H2SO4, and this.
Edit: Man do I feel like a jackass. Acetylene is C2H2. Lousy nomenclature... Grumble grumble.
This is in no way an endorsement of Birch reduction.
(Hive Bee)
02-28-04 05:12
No 491609
Found in wanted references:
Improved synthesis of phenylacetamides by the Willgerodt reaction with microwave heating.
Strauss, Christopher R.; Trainor, Robert W.
Organic Preparations and Procedures International (1995), 27(5), 552-5. http://hyperlab.0catch.com/OPPI_1995_27_
roger2003
(Moderator)
02-28-04 14:00
No 491662
High-yield synthesis from styrene oxide or styrene glycol: G. Paparatto, G. Gregorio, Tetrahedron Letters 29, 1471 (1988)

Chemistry is our Covalent Bond
(Hive Bee)
02-29-04 03:21
No 491762
This compound is used in the converting-process:
P-Tert-Butylcatechol(TBC)
Synonyms 4-Tert-Butyl Catechol,4-tert-Butylpyrocatechol
CAS Number:98-29-3
Appearance: white or pale yellow Flake
Assay:99.5%
Melting Point:53
Crystal Point:52
Boiling Point:285
Ash:0.13%
Packing:30kg Drum
4-Tert-Butyl Catechol is mainly employed in the distillation and transportation of olefin monomer as high efficient retarder. 4-Tert-Butyl Catechol especially suits styrene, butadiene, chloroprene, and chloroethylene . 4-Tert-Butyl Catechol?can also be used as antioxidant of polythene, polypropylene, nylon and other polymers, oil and its ramifications,ethyl cellulose lacquer, lubricants, and metallic soap,etc. Besides, it can serve as passivating agent of the catalyst of carbamater and stabilizer of various kinds of organic compounds.
roger2003