03-03-04 00:59
No 492497
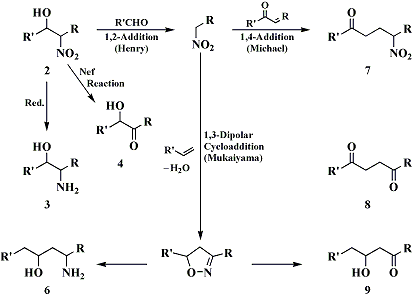
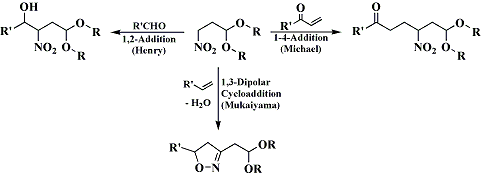
if one were to perform a "henry condensation" between thiobenzaldehyde ,PhC(=S)H, and nitroethane, if a decent yield of the nitro thiol would result, it would seem that this could be reduced with great ease to amphetamine, for example using urshibira Ni at low pressure.
is there any lit. examples of henry condensations with thials?
(Wizard Master)
03-03-04 12:12
No 492614
http://www.orgsyn.org/orgsyn/prep.asp?pr
http://www.orgsyn.org/orgsyn/prep.asp?pr
(Hive Bee)
03-04-04 19:57
No 492975
but these examples all appear to be regular henry condensations, i was looking specifically into henry condensations between a nitroalkane and a thial (thioaldehyde).
(Hive Bee)
03-05-04 01:35
No 493040
Googling for "thiobenzaldehyde" yields a hit, http://www.csj.jp/journals/bcsj/bc-cont/
fear fear hate hate
(Hive Bee)
03-05-04 19:50
No 493200
thanks moo, too bad such an appealing route had to die an early death though.
(Hive Bee)
03-09-04 20:26
No 493997
maybe this method can bee revived with some modification.
for instance by henry condensing phenyl-nitroethane with thioformaldehyde.
the only possible complication i see is that thioformaldehyde exists primarily as a trimer, i don't how that would effect the henry condensation.