03-27-04 23:03
No 497825
Swix was thinking the other day...
A great majority of Alkaloid Extraction Procedures use solvent extractions (using HCL, alcohol etc) on the (powdered) plant matter and then are followed by basing and a NP or steam extraction of the alkaloids.
The passage of alkaloids from the encased plant matter into the solvent depends on a number of things; one of which is the state of the cell wall, membrane and other alkaloid packaging centres.
Wine makers for years have used enzymes to 'clean' crude wine extracts of unwanted biomaterial. The most often used enzymes are Pectin - which breaks apart the links between cell walls; and Cellulase - which degrades the cell walls themselves.
Is there any reason why these enzymes (available cheaply and with very high activity) cant be used as a first-step for alkaloid extraction procedures. Swix's main concern is whether the enzymes will side react or release substances that will harm the alkaloids. And furthermore, whether the enzymes need to be removed or will hinder the steps that follow.
In essence, Swix doesn't see why these enzymes cant be used in the initial soak step to thoroughly degrade the plant so that the alkaloids are released.
He realises this isn't groundbreaking; moreso one of those 'why didn't he think of that before' ideas.
(Wonderful Personality)
03-28-04 12:35
No 497877
Pectin is after the Merck-index not an enzyme but a polysaccharide (a "sugar-polymer") which cements the cellwalls together. This might be not exactly what you are after
Use: In the preparation of jellies and similar products.
It depolymerizes and breaks down under acidic conditions.
I believe cellulase is not what you told it might be too, but I have to look it up.
ORG
(Hive Bee)
03-28-04 12:51
No 497878
referring to pepsin which breaks down proteins, maybe??
wanted: witty signature...pm with details
(Wonderful Personality)
03-28-04 13:17
No 497882
Maybe.
btw. does anybody know which enzymes are in those "septic-tank cleaners" - those enzyme stuff which makes emptying of septics almost unnecessary - at least for a long time? I always had the idea that these enzymes which are able to break down complex organic compounds might be able to produce something useful so feeded with the right starting compounds....
(Hive Bee)
03-28-04 13:46
No 497884
the majority of degredation of matter in septic tanks and other suck treatment devices was carried out by bacteria more so than by enzymes but i could be wrong. i know enzymes are used but the bacteria are more sustainable.
have googled a bit and the sites are mostly commercial and hence, non-informative.
would it not be easier to decide on the substrate first then see if nature has an enzyme to fit the purpose?
wanted: witty signature...pm with details
(Wonderful Personality)
03-28-04 14:46
No 497890
Nature has an enzyme for utmost everything imaginable, but only some are OTC and cheap available like the septic-cleaners which I have used with great success times ago after I had committed genozide to the whole bacterial population of my septic tank where I had lived at this time.
Actually Geezmeister brought me onto this - he recommended the use of those enzyme cleaners once and he was right.
(Stranger)
03-28-04 15:15
No 497894
I believe biotechdude was refering to a pectinase, which breaks down pectin. In fact I'd put money on it because I've used them before to make wine.
In using enzymes for alkaloid extraction, it is a wonderful idea and not sought out as often as it should. Enzymes are a type of protein, and proteins are ubiquitous in nature. In other words they are already in your plant material, just maybe not the right ones.
In brewing beer, the grain/water mix when first added together is a thick syrupy mass which is highly emulsive. The mix is then heated in order to activate enzymes which break up proteins (proteases), starches (amylases), and fats (lipases). After a given amount of time the mixture becomes much less viscous and if allowed to settle will give a clear supernatant above the grain mass instead of the initial emulsified mix. This allows for the solution to be strained for use in beer.
In other words, enzymes added to a plant material (if the right ones were chosen) would break up cell walls (lysozymes and cellulases etc.) and release the contents, and break up starches and lipids that cause emulsions or become carmelized or saponified upon basification before extraction (which is really just continued emulsification). These would not need to be removed any more than the proteins in the plant that are already there should be removed, and who knows maybe it'd make it easier if they were. Of course if you added an extreme excess that would cause some problems, but enzymes are surprisingly effective at low concentrations and are catalysts, so low concentrations just need more time.
It is said to be beneficial to soak morning glory seeds crushed up in water before processing them at all for consumption. There is a cyanogenic glycoside which resides on the outer shell which causes poisoning when the enzymes begin to chew it apart. If the hydrogen cyanide is allowed to escape before consumption then you will not spend an entire night vomiting and having intense stomach cramps. So here is yet another situation in which enzymes are usefull.
I have wondered in the past whether those microcrystalline celluloses are succeptible to cellulases. If so we could exchange a terrible emulsifier for a harmless sugar. Wouldn't that be great?? the enzymes would even be wonderfully dispersed in solution by that crappy ass PEG.
My suggestion: if you are trying to extract plant material, try and grind up the plant as much as possible, maybe by blending it in the water you will extract it with. Then add one of those digestive enzyme capsules you can get at a health food store that have almost every activity (lipid, starch, fat, protein, cellulose, lactose
Last but not least, I wouldn't fear for your alkaloid. Enzymes are tremendously specific in nearly every case and only consume certain types of molocules, and even only certain sterioisomers of the same molocule. Unless you had an ephedrinase, ephedrine would not be touched.
(Stoni's sexual toy)
03-28-04 15:24
No 497899
Enzymes wtf?!?
In the case of beer and wine making you are processing the whole mixture and NOT separating anything. Just because there are enzymes which break down stuff like carbohydrates that does NOT mean that such enzymes would be useful in ephedra extraction, or even pill extraction.
I know that it's hard, but please try not to throw every off-hand idea into a big bucket and stir it real hard like (counter-clockwise, 11.48 times exactly) in the search for a bigger, better mousetrap.
BUSH/CHENEY 2004! After all, it ain't my country!
(Stranger)
03-28-04 17:43
No 497914
Actually, Osmium, you are separating the sugars, amino acids, small proteins, and inorganic nutrients from the grain matter. Separating, and isolating. Sure, it's not one compound you are after in the malt, but you are still after certain things, and those things you can only achieve through enzymes.
Beer is a little different, because enzymes are used to create and liberate the compounds you are after. I stand by what I said though, that it is probably useful, and definitely wouldn't hurt to try breaking down ephedra plant matter in order to free up alkaloid and liquify the mix.
In separating ephedrines from ephedra you are also processing the whole mixture. It is this whole mixture that contains the few compounds you are after. Cell walls, lipid bilayers, carbohydrate matrices and all that jazz likely contain and or bind the alkaloids. In other words, the alkaloid is probably inside the cell, maybe even inside an organelle, and dissolving those things would be suggestible.
"Just because there are enzymes which break down stuff like carbohydrates that does NOT mean that such enzymes would be useful in ephedra extraction"
No shit, just because heating speeds most reactions up doesn't mean it will always help, but hey, it might. In separating psue from other pill gunk we are trying to rid ourselves of both compounds that bind our substrate and those that cause trouble in separating our substrate. If those binding and emulsifying compounds could be removed or broken down, then separation would be easier and more efficient.
I know it's hard, but open your mind to new ideas and don't be so quick to throw them out. Even ideas with flaws can spark someone's mind.
(Hive Bee)
03-28-04 18:10
No 497918
I'm sure I remember a relative of mine purchasing yellow packets in the refrig area of the grocery for the septic tank. Yeast???
What is the first reaction a pill undergoes when one is put in ones mouth? This would explain Dick's hair brained idea w/spit.
As food is digested it undergoes a ph change when passing from the stomach to the intestines similar to raising the ph with soda. Also found a web sight stating blood ph averages around 7.4. Always thought it was more like 6.8. Hmmm. The enzyme area will be imoprtant to any die hard pill fans in the future. Pharmicuticle companies seem to be all about atatching things to amino acids and such for better bioavailability and dose timing.
Where iz that spel chk buton?
(Hive Bee)
03-29-04 02:33
No 497954
it is a well known fact that the enzymes in saliva decompose starches into sugars.
Os: go do some research into digestive chemistry.
it's bloody obvious that if the pill delivers it to our gut that whatever it does will release the precious out of the gakkkkks. so good on yas. I will vomit and spit on everyone's flasks if you want
(Stoni's sexual toy)
03-29-04 03:38
No 497963
I don't think that starch is a major headache in precursor extraction, or is it?
The semi-hydrolyzed components of pills or plant material will cause you much more problems than cellulose and other carbohydrates or proteins which otherwise could simply be filtered out.
If you want to break down cell walls for ephedra extraction then grind and freeze the shit a few times. I wonder why this should be necessary though, with reported extraction efficiencies of 96% and better without spitting on your mormon tea powder.
The new gaak that everyone is complaining about is a modified PMMA which is quite resistant to any enzyme your body might be able to come up with. Dentures are made from PMMA, and I've never heard of dissolving dentures.
BUSH/CHENEY 2004! After all, it ain't my country!
(Hive Addict)
03-29-04 12:51
No 497996
In Case Of Fire Brake Gla........
"HEY...Who Put that Unbreakable Glass Thare?"
"Here...Throw these dentures at it"
Os: They called you a lazy scientist for using that term 'PMMA'!
L░░k! http://www.psrc.usm.edu/macrog/pmma.htm
Acrylic "latex" paints often contain PMMA suspended in water. PMMA doesn't dissolve in water, so dispersing PMMA in water requires we use another polymer to make water and PMMA compatible with each other. To see how we do this, go visit the poly(vinyl acetate) (http://www.psrc.usm.edu/macrog/pva.htm)
Poly(methyl methacrylate), or PMMA, is a hard, tough, and shiny plastic, and if when it forms in the wet paint, it makes the paint surface, hard, tough and shiny. This is good. We want paint to do this. But there's a problem. PMMA is hydrophobic. It doesn't dissolve in water, and a lot of paints are water based.
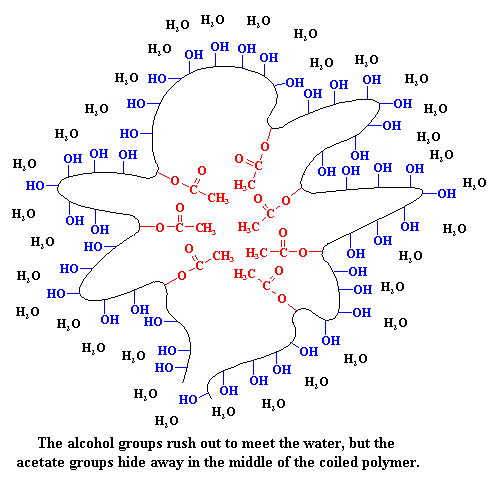
This is where poly(vinyl alcohol-co-vinyl acetate) comes to the rescue. You see, this copolymer has an identity crisis. The alcohol groups are hydrophilic. They love water, and want to dissolve in it. But the acetate groups are hydrophobic. They hate water and don't want to dissolve in it. So when you put the copolymer in water, it forms into a ball. The alcohol repeat units are on the outside of this ball, happily embracing the water, while the acetate groups are on the inside of the ball, hiding from it.
Now ask yourself this question.Haahaaa...I love this partIf you were a molecule of PMMA, and as a PMMA molecule you hated water, where would you go? Would you go out into the water, or would you go into the inside of that coiled polymer, away from the water? You'd go to the inside of the coil of course! And that's just what the real PMMA does. The PMMA hides in the hydrophobic center of the coiled polymer. By doing this, it can stay suspended in water based paints! A suspension of an insoluble substance, like PMMA, held in suspension by being wrapped in another kind of molecule, like our copolymer, is what we call a latex. That's where we get the name latex paint.
Rhodium was right on the money when he coined the term "behemoth" when describing the new gaak amphoteric beasts
Two things here.
First is copolymer permeability via ammonium salts
EUDRAGIT« RL 100 and RS 100 are copolymers of acrylic and methacrylic acid esters
with a low content in quaternary ammonium groups. The ammonium groups are present as
salts and make the polymers permeable.
Second is this...thoughts to ponder!
>I don't think that starch is a major headache in precursor extraction, or is it?
What if they are including amphoteric starches as well?
AMPHOTERIC STARCH
Composition: The word "amphoteric" implies that a chemical product has both positive and negative ion groups when it is dissolved in solution. When papermakers refer to amphoteric starches they are usually referring to products derived from corn starches. The least expensive starch products are based on dent corn, a corn variety that is also the source of most corn sweetener. Amphoteric starches also are prepared from waxy maize, a variety of corn that produces only the branched type of starch, amylopectin. The cationic groups are the same quaternary ammonium substituents used in preparation of cationic starches. The anionic groups usually are phosphates. Strictly speaking all cationic potato starches are really amphoteric starches since potato starch contains about 0.08% phosphorous. The cationic content of amphoteric starches is typically in the range of 0.2 to 0.3% nitrogen. All of these products are delivered to the mill as a dry powder having a moisture content of 10 to 20%.
Function: Enhancement of dry strength. Possibly useful for improving drainage and retention, especially in systems where alum is also used.
Amphoteric Starch (http://www4.ncsu.edu/~hubbe/AMST.htm)
Ohhh....one more thing that may come into play soon is the subject of one of Jacked's threads started in General or Chemistry Discourse Forum involving oil and water miscibility modifiers.
a little less conversation, a little more reaction
(Stoni's sexual toy)
03-30-04 01:22
No 498066
And your point is... what? Dunno what you are trying to say.
BUSH/CHENEY 2004! After all, it ain't my country!
(Hive Addict)
03-30-04 18:31
No 498201
Os: Now you know better than to ask me, of all bees, a question like this
>And your point is... what?
You do mean besides the one off the top of my head dontcha...?

My point? Permeability for one, aside from poking holes in your theory concerning extractability of OTC pills nowadaze either naturally(digestive) or solvent/organic based extraction.
Your claim, or as I read it, that modified PMMA wouldn't pose any threat due to it's impermeability doesn't hold water in my eyes
I felt it would benefit the bees if they saw some of the dynamics that go into some of the more sophisticated OTC pill engineering of today and some of the twists and turns they will need to consider if they intend to duplicate any digestive assisted extraction approach. Those Do-bees with an above average capacity to conceptualize processes beyond the conventional norms will digest the information provided and the points made. Those lacking in the creative imagination department need not apply
With any luck, the points made may just spark some much needed "craziest shyte" discussion/experimentation in order to thrust some solution our way.
For the longest time I've had the feeling, judging by some of your remarks that you prefer to downplay the threats that bees are confronted with, concerning the newest foilants and denaturant/adulterants.
It's always been my aim to expose the areas that may cause the biggest threat without exaggeration or discouragement.
While I agree that this thread traveled off the intended mark(enzyme assisted Alkaloid extraction) somewhat, midway down with the comparing of apples and oranges, I thought I'd chime in with some facts of enlightenment how they may relate to areas such as the ones you addressed in your replys.
Being a 3-D bee...I never have just one point. Multi-dimensional thinking and contemplation demands more.
For those that think it's torture having to endure the ADHD types around here, they should try the affliction on for size for just fifteen minutes before casting stones.
Even the least significant areas of study concerning co-polymer research require an equivalent amount of thought and examination as the number of minds that had a hand in their creation. Yes we fall extremely short. For this reason we operate without the same constraint that governs them by exploiting every concievable avenue that might show promise. Being openmindedly willing and able to exhaust every single possibility without prejudice or predilection is what sets a Do-bee apart from an average bee driven by preconcieved conditioning or notions.
Just for shytes and giggles...
And some experimental chemistry, I wonder how many bees pondered fighting fire with fire... Instead of spitting on it?
Orange Gaak may have an allergy....
to oranges???
What's that you say?
"He's gone and bumped his head again!!!"
On the contrary...
For instance...This ideology stems from looking at this from a semi-natural extraction approach and is a far cry from duplicating the bodily functional ideas.
This may hold more apPEEL to those on the recieving end of the end result as well.
Instead of slobbering in yer alcohol, why not make a cocktail???
A "Screwdriver" if you will
Instead of using orange juice, try charging your alcohol with orange PEELS before alky extraction.
Does it work?
Ibee don't know!
I said it's just for shytes and giggles.
Do some research and try it!
For those with nothing to lose...whatta ya got to lose?
Bee thankful I didn't suggest a "Bloody Mary"....
a little less conversation, a little more reaction
(Wonderful Personality)
03-30-04 19:52
No 498218
Of course, saturating ("preloading") the alcohol with another soluble compound to hinder the "orange gakk" to get dissolved is a good idea - and absolute not lunatic but following the laws and theories of extraction techniques. Actually this is a very common technique in industry.
On the other side this has nothing to do with enzymes - and Ware forgot to name the name of the compound to use for preloading the alcohol....
Not my problem though...
summary:
- enzyme for ephedra extraction: nonsense as not necessary
- enzyme for pill extraction: this question has to be answered by the highcouncil of the several churches of pill extraction.
- are OTC enzyme useful in clandestine chemistry: this is unanswered and would be most interesting IMHO....
(Hive Bee)
03-31-04 18:26
No 498386
http://www-saps.plantsci.cam.ac.uk/docs/
"...Protoplasts are cells which have had their cell wall removed, usually by digestion with enzymes. Cellulase enzymes digest the cellulose in plant cell walls while pectinase enzymes break down the pectin holding cells together...".
http://www.accessexcellence.org/AE/AEPC/
"...Macerase is Sigma's proprietary name for a crude pectinase obtained from the fungus Rhizopus; pectinase separates cells from one another.
Cellulysin is Sigma's proprietary name for a cellulase isolated from Trichoderma uride. This enzyme degrades cell walls..."
Now protoplasts are VERY suseptible to breakage; through osmotic, heat or mechanical shock (by stirring 11.48 turns Os).
As anyone that has done alkaloid extraction knows, you can use a variety of techniques to powder up the plant and rupture cell walls. HOWEVER; come extraction time, these fine plant particles tend to rejoin and gum up together, hindering the passage alkaloids into a NP or steam flow; or trapping them altogether. SO rather than force cells apart, you can just degrade them altogether...
In regards to the reported claims of 96%; this may well be true. However, these claims were calculated from the manufacturers claim of 8% alkaloid content (which +/- varies greatly) and were achieved using laborious procedures. Furthermore, the ability to reproduce such results consistently are questionable so enzymes were suggested in the interests of quick, reproducable larger scale extractions.
Reflux's extraction suggestion is right on the money. In summary - fine powder plant, cover with water, add enzyme mix and heat to 37`C and stir, make acidic, separate acidic (press, decant), make basic, NP extraction or steam distill.
Re pill extraction and enzymes, Swix will follow that one from the sideline. But he loves that his initial post (which he assumed wouldn't get much response) has flowed into this topic area.
(Stranger)
04-01-04 00:12
No 498434
Erm.. Hi.. SWIM does a bit of tissue culture..
Ears pricked up when he read protoplast. Enzymatic reactions to protoplast, this procedure can be emulated much faster chemically by the addition of polyethylene glycol to the plant cells. It's how tissue cultures get thier protoplasts to do fusion techniques and so forth.
(Stranger)
04-11-04 01:58
No 500174
my alter ego, pancuronium, raised the question of enzymes by pondering saliva. He was ridiculed and discounted.
enzymes seem very promising even for the matrices of tomorrow. Keep up the good brain storming and we will beat the enemy by those of us who think outside the box.
(Stranger)
06-15-04 06:16
No 513548
the septic cleaner you refer to is showing limited promise but ive done limited studies. be sure to post any work you may do to prevent wasted effort and retraced steps.
To the lab!