08-08-02 05:22
No 342810
http://www.sfu.ca/~lower/critical.html
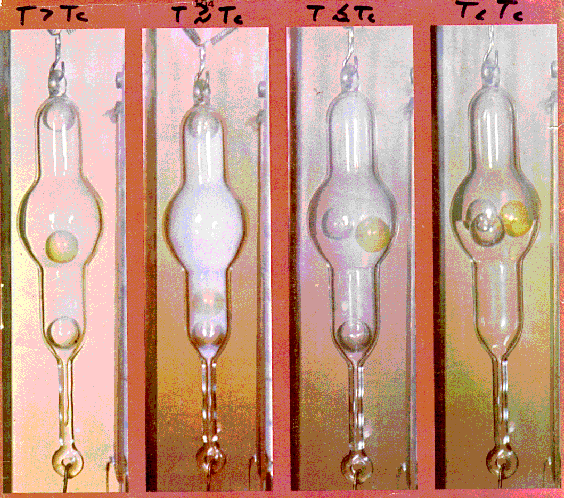
The 4 photos show a glass tube filled with CO2 at about 1000 psi and at temperatures above, around and below the critical temperature. There are also 3 ball floats in the tube having densities just above, around and below the density of supercritical CO2.
The left photo shows the tube completely filled with supercritical CO2. The float with the density of supercritical CO2 is floating in the middle because while supercritical CO2 has the density of a liquid it is compressible like a gas and thus the effect of gravity sets up a density gradient in the tube.
The 2nd photo from the left shows the transition from supercritical to liquid. The intense opalescense is caused by scattering of light by the mixture of the two states.
In the 3rd photo from the left there is no supercritical CO2 left just liquid and gaseous forms of CO2. Two of the floats are floating on the surface of the liquid CO2.
In the 4th photo the temperature has been decreased a bit more causing an increase in the density of the liquid CO2 and now all three floats are floating at the liquid-gas interface.
(Hive Bee)
08-08-02 05:26
No 342813
Cool~~ swim didnt think liquid CO2 existed.
hcildoduedn
(Distinctive Doe)
08-08-02 08:21
No 342886
What do you think is in a cylinder of CO2?
It ain't gas and it ain't soild.
Those who give up essential liberties for temporary safety deserve neither liberty nor safety