10-07-02 18:23
No 365741
After SWIM experienced the joys (NOT!) of gassing, you know the wonderful clouds of HCl wafting thru the summer air, he is determined to find a "kinder gentler" route to end product.
After looking through the FSE, have come up with the following and would like opinions, other ideas:
Post MM Al/Hg: Combine A/B with Titration
1) Extract Xylene with 2x 100 ml 10%HCl.
2) Basify with 30% NaOH.
3) Extract with 150 ml Xylene.
4) Extract Xylene with 100 ml 10% HCl.
5) Evaporate H2O.
6) Recrystalize from dry IPA.
Comments? Suggestions?
If they drive God from the earth, we shall shelter Him underground - Dostoevsky
(Hive Bee)
10-07-02 18:52
No 365754
What was your problem with gassing??? Swim loves gassing. He even has a little dance he does right before he collects his crystals. What was the problem?? Im sure if you did it right, you too would be happy.
& I'm, somethin of a phenom, no need for da cron, I'm un-stopable.
(Title on BackOrder)
10-07-02 18:54
No 365756
extract with toluene/xylene as usual, acidify with hydrochloric acid, basify and extract with DCM, strip off DCM, fractionally distill, take up in IPA, neutralize with concentrated HCl, crash out with ether, filter, wash with ether.
All paths are the same: they lead nowhere
(Hive Bee)
10-07-02 18:59
No 365758
Maybe extract with HCl(aq), basify, extract with NP, vac distill FB, dissolve FB in IPA, titrate with HCl(aq), vac distill off a large portion of IPA/H20, dump in 2-3x volume of acetone, freeze, dump in buchner. Vac distill off more IPA/H20 from filtrate, dump in more acetone, if any more crystals, dump in buchner.
Who is that masked man?
(Hive Bee)
10-07-02 19:29
No 365772
But not for me! You can't do it indoors; No matter how careful/slow you do it, some residual HCl gas is wafting around, dissolving the finish on my hotplate and my lungs. And you're left with a nasty acidic salty mess to clean! And that Sulfur smell - hmmmmmm. Just keeps on going and going....
If they drive God from the earth, we shall shelter Him underground - Dostoevsky
(Chief Bee)
10-07-02 19:42
No 365777
You've made 1.25 gram MDMA crystals? What was the crystal shape?
(tourbee)
10-07-02 19:52
No 365783
hell yeah diggity!You do that dance too!And Max send a double terminated crystal this way.Need it for a crystal healing configuration.
You can tell the queen of diamonds,by the way that she shines.-GD
(Title on BackOrder)
10-07-02 20:04
No 365790
I've had one gram crystals form before, and they were rectangular in shape, clear, and beautiful to look at. It almost made me cry to have to take a razorblade to them.
All paths are the same: they lead nowhere
(Greenhorn)
10-07-02 20:38
No 365802
A very simply way to get your crystals is as follows. Work up your MM al/hg as usual. Do all of your washes on your tol or xylene. Make 5-10% acidic water. Put your xylene/toulene in a sep funnel and add your acidic water. You can watch the mdma freebase migrate to the water layer. Shake a few times and let seperate. Tap off water layer and evap till you get a syrup. Let cool and add a bit of acetone. Crystals everywhere. Clean as usual. Don't thank me, thank noj.
I went into the business for the money, and the art grew out of it.-Charlie Chaplin
(Hive Bee)
10-07-02 23:14
No 365844
http://146.201.224.61/pharmaceuticals/pa
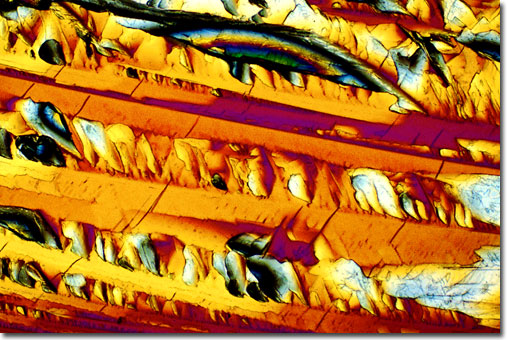
MDMA under a scanning-electron microscope.
Tis' an ill wind which blows no minds
- Discordia
(Hive Bee)
10-08-02 00:50
No 365893
Gosh, and they said MDMA is not a psychedelic...
Mountain Boy
(Hive Adickt)
10-08-02 01:12
No 365901
Bubbleplate, back to the subject. Iff you make a solution of HCl in ether or alcohol you just drip this solution into your dry toluen/frebase until your wet pH paper shows that the solution is acid. Can't bee easyer than that.
Making the stock solution is a one time job.
(Hive Bee)
10-08-02 05:50
No 366008
BTW, SWIM got 14g honey on first try - probably due to weak nitro. Feels good to have gone the whole route. Now can improve technique.
If they drive God from the earth, we shall shelter Him underground - Dostoevsky
(Chief Bee)
10-08-02 08:28
No 366056
Would it be possible to grow several uniform crystals? Wouldn't it be a very cool to grow crystals, aiming for a weight of 120-130mg, and then use single-crystal MDMA.HCl as distribution form? That would be way better than pills or capsules not only from an aesthetic point of view - who could cut or otherwise tamper with a uniform crystal? A clear rectangular crystal of MDMA.HCl always has the same size, so people would know how big it would suppose to be... Any takers?
(Hive Bee)
10-08-02 09:00
No 366066
Hest, would you have to gas to get that stock solution? Could there be a way of doing it by first mixing regular 37% HCl w/ alcohol or ether then removing the water chemically, for example using CaO or anhydrous something or other?
(Hive Bee)
10-08-02 09:02
No 366067
growing "Rock Candy"? i.e. make a supersaturated solution and put in a chamber that had weighted cotton threads suspended in it. Simple. Maybe even roll the wetted threads in some fine powdered material first to have some starting points for the crystals.
Granted the crystals wouldn't all be the same size, but tweaking the solution strength you might get fairly consistent sizes. When done, cut off the individual crystals.
If they drive God from the earth, we shall shelter Him underground - Dostoevsky
(Chief Bee)
10-08-02 09:26
No 366075
Madmax: I don't have any links up my sleeve at the moment, but there are loads of crystal growin sites available on the net.
Genius: Acros and others sells 5 M HCl in IPA very cheap.
(Hive Bee)
10-08-02 09:39
No 366078
Rhodium-- I know, however, it would be much better if it could be made at home, especially since I do not have an account with Acros or any of the big domestic chemical companies. I only have access to smaller chemical companies that do not carry HCl/IPA or HCl/MeOH, etc, and also access to some overseas chemical companies, and shipping corrosives internationally is expensive. It would be nice to know how Acros, etc. make their stock solutions. Do you think the method I described would work at all with any "water-removing" chemical? I am very interested in this topic because I hate gassing and have burned my lungs numerous times and also my skin even with gloves on. Not only clandestine, but in the academic lab.
(Hive Bee)
10-08-02 09:49
No 366083
How is the 5-6 N HCl/IPA from acros made? By gassing!!
Adding CaO to wet HCl in IPA would be a good, but expensive, way to get CaCl2.
Why donīt you simply follow more experienced bees advice and devote one, just one, day to make your stock solution of HCl in whatever-solvent?
Catalytic hydrogenation freak
(Hive Bee)
10-08-02 09:55
No 366085
Well, HCl salt is an option, but surtenly not when it comes to amphetamine (european bees know what I'm talking about). Why? Becouse amphetamine.HCl is highly hydroscopic and the street name for the amph.HCl overhere is "goo-speed"... Anyways, phosphate salt is to prefer, this is a piece of information taken from rhodium.ws that can bee useful:
"....135 g (1 mol) of amphetamine freebase were stirred into 300ml of acetone in a 1000ml erlenmeyer flask. To the resultant solution there were slowly added under constant agitation 115.3 g of 85% phosphoric acid (containing 1 mol of H3PO4), care being taken to avoid any sudden rise in temperature or local overheating due to the considerable amount of heat that is evolved. During the addition of the phosphoric acid a fine, white, flocculent precipitate appears which becomes more and more dense and abundant, as the quantity of added acid increases.
When the entire quantity of the phosphoric acid has thus been added, agitation of the mixture is continued for about a half-hour or more to insure complete conversion. The precipitate is then allowed to settle, the supernatant liquid is drawn off, and the residue is filtered. The precipitate thus separated is washed with acetone and is then dried by evaporation to constant weight. It forms a fine, white, impalpable powder consisting of pure monobasic amphetamine phosphate.
Reference: Pharmaceutical Manufaturing encyclopedia (1988)..."
[pH]armacist - Never underestimate the power of retrosynthesis. Svenskar suger på fotboll!
(Hive Bee)
10-08-02 10:00
No 366087
True, very true! There are other acids to use besides HCl. Try citric-, acetic-, tartaric-, phosphoric- and sulphuric acid, to name a few.
Catalytic hydrogenation freak
(Hive Bee)
10-08-02 12:13
No 366124
Hows about sticking your mix in a soda pop carbonation device,such as a soda stream.Cheap,clean,efficient.
Seems like it was designed for this purpose.
Does carbonate form with MDMA?
(Chief Bee)
10-08-02 12:24
No 366131
Yes, MDMA carbonate can form. Carbonates are pretty unstable salts though.
(Hive Bee)
10-08-02 13:11
No 366158
But the soda pop carbonation device won't produce carbonic acid on its own, the carbon dioxide has to react with water first. Theoretically there has to be one mole of water per two moles of an amine freebase. (CO2 + H2O --> H2CO3)
(Whisperer)
10-08-02 14:22
No 366192
To the original poster: ever tried to UTFSE?
When all you've got is a nailgun, every problem looks like a messiah...
(Chief Bee)
10-08-02 14:42
No 366203
Madmax: Yes, as methamphetamine carbonate decomposes to the freebase upon heating, smoking it is essentially like smoking the freebase, i.e. stronger, faster, etc., yet it is still a convenient to handle solid.
(Hive Addict)
10-08-02 15:57
No 366228
With MDA, you could obtain the ascobate using Vitamin C which is supposed to absorbed by the body quicker, and be more potent (see Post 59321 (LaBTop: "Re: MDMA chirality", Serious Chemistry)).
The above post is purely fictional. Any resemblance to "real-life" is purely coincidental.
(Hive Bee)
10-09-02 02:13
No 366413
if you distill ether from starting fluid, it is not a good idea to store it too long. When distilled, the ethers (normally a mixture of diethyl/di-isopropyl) will have no stabilizers, and will be prone to the formation of explosive peroxides. It is suggested above to store it in the freezer. This may or may not help, I have no idea and would not like to find out myself.
Also be aware that storing extremely volatile solvents in non-lab freezers can create powerful explosions if the solvent vessel is not absolutely air-tight. Otherwise solvent can escape and be sparked off by the thermostat.
(PVC-Analog Taste-Tester)
10-09-02 04:30
No 366437
In my dreams, I've had the acetate and hydrochloride of MDMA; both kick in at approximately the same time. One could always dissolve the freebase in a tiny bit of glacial acetic acid, then put a fan on it to dry, but you might want to make plans to leave it behind a closed door for 2 days unless you don't value your mucus membranes, lungs, and eyes. If the freebase was dissolved in toluene or ether, then acetic acid slowly dripped in, would the goods precipitate out?
Love my country, fear my government.
(Hive Bee)
10-09-02 13:20
No 366587
Maybe...
One (very old) method is to simply shake ether (ether must be used) with concentrated hydrochloric acid in a sep funnel. Most of the HCl will dissolve in the ether by forming the oxonium compound, creating considerable heat, so expect to vent your sep funnel very often! The water phase (lower phase) is now drawn off and discarded and the ethereal HCl solution (upper phase) is saved. All you have to do now is to titrate a ethereal solution of your amine with the ethereal HCl solution to get the hydrochloride.
As I know from personal experience this methods works best with
a) amine hydrochlorides which are not too hygroscopic and
b) lots of ether used for the amine solution
because the HCl/ether solution contains still some water, which will stay dissolved if lots of ether are used.
This procedure works for sure with some alcaloids (atropine, quinine), so you could give it a try on a small scale for your preferred "alcaloid".
Quidquid agis, prudenter agas et respice finem!
(Chief Bee)
10-09-02 13:38
No 366592
I think that ether usually dissolves 7-8% water.
Alcohol, an unconsciousness-expanding drug
(Hive Bee)
10-09-02 18:02
No 366678
So if CaO + HCl __> CaCl2 + H2O, then what happens when you add a buttload of anhydrous CaCl2 to a solution of alcohol or ether and 37% aq. HCl? Does it remove the water?
(Chief Bee)
10-09-02 18:14
No 366684
Yes, it will absorb water, but as HCl also is hygroscopic, I don't know which compound would pull hardest and keep the water. CaCl2 will catalyze the chlorination of alcohol to the alkyl halide though, causing a mess.
Alcohol, an unconsciousness-expanding drug
(Hive Bee)
10-10-02 00:55
No 366781
IF YOU ARE HAVING PROBLEMS GASSING.. LET ME HELP!!
After fuckin around, I found this to be factual, and interesting.
Swim has a bubbler setup, and noticed something interesting a few weeks back.
He had 2 containers in which he was preparing to gas 1600ml's of nonpolar/freebase
One of the containers was a large 2L pyrex measuring cup, and the other, a 1L beaker.
Each were filled with 800ml's of the nonpolar/freebase.
Using the exact same bubbler setup on both gassing runs, the 1L beaker filled with crystals wayyyy faster then the 2L meauring cup.
This leads me to believe that the taller and slimmer the vessel is, and the lower that gas distribution tube is, in the non polar, the faster your shit will get done.
I think it has to do with the hcl gas bubbles traveling upwards through the non polar crashing out more crystals than if the tube was 10mm into the solution, sitting in a lowform dish, like the 2L pyrex measuring cup.
This may help some, this may go unheard, you may not give a flying fuck, either way, it works for sure. Swim tries his hardest to make sure no variables are changed when he experiments. The 1600ml's were from one batch, just split in 2.
Each produced about same amount. One was just done faster. I bee on the hunt for a super thin, very long/tall vessel to use.. experiment further..
& I'm, somethin of a phenom, no need for da cron, I'm un-stopable.
(Hive Bee)
10-10-02 01:08
No 366785
I only have access to smaller chemical companies that do not carry HCl/IPA or HCl/MeOH
When SW is gassing 2000 ml IPA w HCl w stirring, he can gas it quite a bit before there's any residual fumes at all. It does fume a little bit when the solution is poured but it's a much nicer system than gassing product disolved in toluene or ether fume wise.
(Hive Bee)
10-10-02 01:16
No 366789
If you do shit right, and got a lab separate from your home, and it's out in the middle of god know's where! LIKE IT SHOULD BE, then it's all good, in fact! It's all right!! You know the rest.. fuck all day.. fuck all night, and gas in gas in peace. Just set up a table by a window with the fan blowing out and you can fall asleep next to it and not smell a damn thing.
Swim has never gassed anything but toulene, so he wouldn't know the joys of gassing ipa..
He would like to titrate into dcm someday. He really enjoys smelling dcm. It's so bubblegumish. Makes him happy when he smells it.
& I'm, somethin of a phenom, no need for da cron, I'm un-stopable.
(Chief Bee)
10-10-02 02:26
No 366816
HCl gas corrodes everything in a matter of minutes - I hate working with it. It even discolors stainless.
HCl in DCM? I wouldn't reccommend it for amine crystallization, as many salts (like MDMA.HCl) are partly soluble in DCM.
Alcohol, an unconsciousness-expanding drug
(Hive Bee)
10-10-02 15:54
No 367033
than it was worth, she had planned on using one of those "Pyrex Gas Washing Bottles". You know, the kind that has a removable top and is taller than wider. Plus it has an Inlet to gas the product, and an Outlet that could be run to a basic solution or vented.
Oh yeah: someone posted that they had inhaled HCl gas. ALWAYS keep a bottle and rag of Ammonia (dilute!) around. If you accidently inhale, it could save your lungs. Breathe a little in.
I've seen plastic Keck clips around GG joints crumble to dust from HCl gas...
If they drive God from the earth, we shall shelter Him underground - Dostoevsky
(Hive Bee)
10-10-02 16:32
No 367049
Wow, you guys have serious issues with HCL gas.. it doesn't bother me any. I always set up shop next to a window with a fan blowing out it and nothing ever gets fucked up, especially my lungs.. I can't even tell im gassing anything, except fot the sound of the bubbles.
My only complaint is the length of time it takes to reach completion.
1600 ml's of freebase containing 50g of product takes a good hour to finish..
Ive heard bee's say they gassed half that amount in 5-10 minutes..
Maybe it's the power the dual headed fish tank pump is providing. Not enough? Not bubbling hard/fast enough? Will get another pump, and add 2 to the mix, maybe that will cut the time in half..
& I'm, somethin of a phenom, no need for da cron, I'm un-stopable.
(Hive Bee)
10-10-02 16:45
No 367059
Swich just sets up the flask that the prodcut is being gassed in with a two hole stopper, and insterst glass rods trough both holes, and then attaches tubing to the two tubes. One of the tubes is a J tube that runs into the product (or a gas dispersion tube) and then other only goes partially into the flask. A tube is run from the outlet tube to the outside through a window, hence all the hcl gas goes outside. Whats so hard about that? Just bee careful on a still cold night in an urban area. Swich has a bad memory of a doing this in a downtown apartment in a metropolotin area, and seeing an entire city block covered in HCl fog from only 30 minutes of gassing. Let me tell you Swich was suprised as hell when they walked out the back door of the apartment and saw and urban street covered in a fog on a 10C (50F) night. Swich has since learned not to do such things in apartments, but if you are an apartent bee, and trying to gas, using this tube venting method be forewarned . . . it's probably the same with a fan, but Swich has never used a fan, always done it this way, until Swich had a fume hood, and then the tube was routed into the fume hood, and it carried away all the hcl gas. Either of these methods wil make almost 0% hc gas onto the reaction area, with no smell. Bt then again every now an again a stopper will blow out, if you don't seucre it properly. Thats when it;s nice to be using the dripping hcl into H2SO4 method of Hcl production. You can just turn it off. If anyone is in the lab with you when the stopper blows off when you are gassing I gaurantee they will FREAK out -- hehe at least thats my experience.
(Stoni's sexual toy)
10-10-02 17:31
No 367085
> Maybe it's the power the dual headed fish tank pump is providing.
Maybe a fish pump bubbling air through HCl isn't the best and fastest way to do this?
I'm not fat just horizontally disproportionate.
(Hive Bee)
10-10-02 17:32
No 367088
Maybe Os is right..
& I'm, somethin of a phenom, no need for da cron, I'm un-stopable.
(Stoni's sexual toy)
10-10-02 17:33
No 367089
Never know until you try...
I'm not fat just horizontally disproportionate.
(Hive Bee)
10-10-02 17:52
No 367093
Try what!?
I was thinkin.. I have this Gast piece of shit I use for vac filterations.. it has an dual head, intake and exaust.. I could hook it up to the exaust and get 100x the power, but that may be toooo fast.
I guess I'll never know till I try..
& I'm, somethin of a phenom, no need for da cron, I'm un-stopable.
(Hive Bee)
10-10-02 20:35
No 367132
SWIM was visiting a friends lab during gassing and they had dripped way too much H2SO4 into the HCl & NaCl. Next thing, foam galore and suddenly not only is a Stopper blowing out, but a stream of HCl and conc. H2SO4! Luckily SWIM had a full face shield, hat, and Kevlar jump suit and not just goggles and dungarees!
Newbees, are you taking notes?? Bee safe! Your eyes and face are worth $100 of protective gear.
(Hive Bee)
10-11-02 01:50
No 367239
H2SO4 dripped onto NaCl
Wouldn't that produce mdma sulfate though?
As soon as he gets a rotovap he will distill his freebase and gas a smaller volume of non polar, rather than gassing such a large amount.. I don't know if that will speed up the process, but it cant hurt..
Would adding some NaCl to the bubbler containg the hcl produce more hcl gas, than without??? Swim has yet to try that..
& I'm, somethin of a phenom, no need for da cron, I'm un-stopable.
(Hive Bee)
10-11-02 02:14
No 367248
Stick a plastic tube up yer butt and bubble away.
You might want to use a trap or check valve, beecause toulene up yer ass is no fun, trust me.
This is what's been done,all jokes aside, titurate the freebase solution (with hydrochloric acid)and azetropically distill off the water as a toulene azetrope, watch crystals appear. works just almost as well as gassing, but SWIS likes to gas , SWIS uses a coke bottle, drill two holes in the cap, fill with salt and HCl wrap teflon tape around the threads, place cap, use rubber as a 'gasket' for your tubing, fill a basting syringe with sulfuric acid, and drip onto the Hcl salt paste, gas away.
This thread should be in the newbee forum.
(Title on BackOrder)
10-11-02 08:15
No 367322
Wouldn't that produce mdma sulfate though?
No, and you seriously need to learn a lot more before you hurt yourself.
All paths are the same: they lead nowhere
(Hive Addict)
10-11-02 10:57
No 367363
so is the idea with the hcl in the IPA, that you heat the ipa to liberate the hcl, or do you drip the IPA into the freebase/solvent mix?
Infinite Radiant Light - THKRA
(Hive Bee)
10-11-02 14:24
No 367430
Diggity,
H2SO4 + NaCl ---> Na2SO4 +HCl
The HCl used to gas the mdma freebase to mdma.hcl
To bee or not to bee, that IS the question.
(Hive Bee)
10-11-02 15:11
No 367439
hidden cloud:
according to argox, it's NaCl+H2SO4 --> NaHSO4+HCl.
No shit, this should really be in the newbee forum. However, I would like to know bees' opinions of the shelf life of various stock solutions of NP solvents and HCl. Namely, IPA, MeOH, Et2O, acetone, etc. They can't sit around forever, the HCl would react.
Who is that masked man?
(Hive Bee)
10-25-02 12:47
No 372645
OK, swim has decided to try Argox's method as outlined on Rhodium's page. He has some questions though. First, can the trap be eliminated if one just uses an inverted funnel in the receiving flask? That's what we do in analytical chemistry to prevent suckback when distilling ammonia into HCl, would it work in this case? It would make the apparatus much simpler. Also swim doesn't have any of those fancy "custom 3-way adapters." Could he just use pinch clamps on the hose? Another thing. Swim just wants a mole of HCl (36.46 g), which should be enough to react w/ 192.7 g MDMA freebase or 178.7 g MDA freebase. What would be a good amount of IPA to dissolve one mole of HCl at room temp? Rhod said they sell 5 M right? so 200 mL should be fine? Anyways, how would I standardize my solution when I was done? Just like if it was aqueous HCl? Or differently? One more thing, does a 5 M solution of HCl/IPA fume at all? And is it stable at room temp or should it be stored at a low temperature?
(Chief Bee)
10-25-02 13:36
No 372657
If you put pinch-clamps on your HCl gas tubing, make sure that any over-pressure inside the apparatus doesn't blow a stopper out (or worse). Use at least 250ml IPA to dissolve one mole of HCl, and chill the IPA with ice when gassing it - the solubility goes down fast above room temp. Standardization of the solution is probably best performed by diluting a sample to 50% with water, add some bromthymol blue indicator and titrate with 0.10 M sodium hydroxide until the color changes. 5M HCl in IPA fumes violently, at least as much as conc aqueous HCl. Room temp is okay, but the cooler the better. Never fill your storage flask more than 2/3 full, and expect overpressure every time you open it.
(Chief Bee)
10-25-02 20:42
No 372748
Chill your alcohol solution thoroughly, or else some of it will evaporate during the exothermic dissolution of the HCl, making you miscalculate the concentration afterwards.
(Hive Bee)
10-25-02 23:00
No 372785
To get the maximum amount of HCl gas absorbed in the freebase/alcohol/ether, you want to use a tall, slender vessel with strong agitation. The taller the vessel is, the more contact time each bubble of HCl has with the solvent, so more mass transfer can occur. Agitation breaks the bubbles into smaller bubbles, disperses them throughout the vessel, and increases the concentration gradient around each bubble. All of these increase the rate of HCl absorption. Fast stirring is absolutely essential to getting higher yields based on the amount of HCl gas used.
(Hive Addict)
10-26-02 03:25
No 372831
so could an ultrasonic agitator be used?
Infinite Radiant Light - THKRA
(Hive Bee)
10-26-02 10:45
No 372914
Ultrasonics are used to degas solvents. You would end up with what you started with.
(Hive Bee)
10-26-02 17:02
No 373024
Anyone have an opinion on getting rid of the trap and using an inverted funnel? Also, how can I analyze the water content of the final solution? Is there any special instrument that is used for this?
(Hive Bee)
10-26-02 17:24
No 373031
I guess if you just wanted to know if there was any water present at all, you could just take a sample of the solution in a test tube and add some anhydrous cobalt chloride, which will turn fro blue to pink in the prescence of water. If you're looking for the amount of water in solution, I have no idesa what you could do.
Who is that masked man?
(Hive Bee)
10-27-02 04:09
No 373192
If water is present it will appear turbid, and in some solvents you can correct this by adding something that will azetrope with water, and then distill the azetrope off through a dean-stark (or whatever).
You would want to use this azetrope method if you goofed during an extraction, and did'nt want to use drying agents for fear of precipitating your good-stuff onto the drying agent as it absorbs water.
(Hive Bee)
10-28-02 06:34
No 373621
Like maybe a Karl Fischer titration or something, I know it's used to measure moisture content but don't know much else about it. Anyways, I'm gonna give it a crack using the inverted funnel maybe later this week since no one seems to have any major objections to that approach. Anyone want to give me some info on the KF titrations? I see in my catalog a Denver Instrument Cuolometric Titration Controller for over $2000 that is for KF titrations. Does KF titration have to be that expensive? Damn!
(Hive Bee)
11-25-02 08:02
No 383198
How is the 5-6 N HCl/IPA from acros made? By gassing!!
Adding CaO to wet HCl in IPA would be a good, but expensive, way to get CaCl2.
Why donīt you simply follow more experienced bees advice and devote one, just one, day to make your stock solution of HCl in whatever-solvent?
There is another way that does not involve any gassing at all. Dissolve acetyl chloride in cold anhydrous methanol.
CH3OH + CH3COCl --> HCl + CH3COOCH3
The byproduct is methyl acetate. Perhaps there is some way to remove it, or maybe it does not interfere with our desired reaction?
(Stoni's sexual toy)
11-25-02 15:13
No 383303
Solutions of hCl in alcohols aren't inert, they will slowly react with each other producing the alkyl halogenide.
I'm not fat just horizontally disproportionate.
(Hive Bee)
11-25-02 17:46
No 383341
Will the alkyl halogenide (alkyl halide?) react w/ MDMA/MDA/2CB freebase at all? Also, how long does this take to happen? I was under the impression that a lot of people gas their solvent and store it until they have freebase, which can be a long time. That's why I like this method, because you can do it on the spot at any time without setting up a gassing apparatus.
(Stoni's sexual toy)
11-26-02 00:48
No 383430
> But does that matter?
Yes, sometimes it does. I've seen examples, e.g. a report by some industrial chemists who tried to prepare a methylester by saturating the methanolic solution of the compound to be converted with HCl gas. While this reaction apparently gave excellent yields and purity in rather short time when done small scale, it didn't work very well when scaled up to industrial proportions, leaving an almost impossible to clean up and difficult to filter mixture after several hours reaction time. Nobody could explain the differences until they found out that the HCl converted some of the MeOH into MeCl which reacted with another site of the molecule, producing impure shit.
> Will the alkyl halogenide (alkyl halide?) react w/
> MDMA/MDA/2CB freebase at all?
Yes.
> Also, how long does this take to happen?
The side reaction starts as soon as you stick the gassing tube into the alcohol.
> I was under the impression that a lot of people gas their
> solvent and store it until they have freebase, which can
> be a long time.
I wouldn't store it for too long. I have no details, but I assume that MeOH and EtOH are much more perceptible to this kind of side reaction than e.g. IPA.
I'm not fat just horizontally disproportionate.
(Hive Bee)
11-26-02 15:10
No 383714
What would the reaction be if you had an amine freebase such as MDMA and an alkyl halide in solution? What would the product be? Also, how about methyl acetate, would it react with an amine freebase? If so, what would the product be? MDMA acetate?
(Chief Bee)
11-26-02 17:27
No 383750
Methyl acetate may acylate MDMA to N-acetyl-MDMA, and methyl chloride may alkylate MDMA to N-methyl-MDMA (N,N-Dimethyl-MDA).
(Hive Bee)
11-26-02 21:47
No 383813
OK, here's another question. What would happen if one were to distill HCl/MeOH solution? Would the HCl/MeOH come over together, or would the HCl come over first then the MeOH? I know that NH3/water comes over first the NH3 then a NH3/water solution (at least that's what we did in analytical chem when analyzing ammonia content in an unknown). The reason I ask is maybe methyl acetate can be removed by distillation?
(Hive Addict)
12-03-02 01:45
No 385782
in the interest of... well... me, a test was performed to determine which yield better results, gassing or titration. less than expected results has become a trend lately, and has cast a dark shadow over the lab. now, before you yell NOOB!, drying and gassing are not new experiences hereabouts, and proper yields have been accomplished regularly in previous attempts. investigations were made, apparatus cleaned and replaced, but to no avail; the spate of poor gassing yields continued. so, this experiment was attempted to decide if methodology should be changed. titration has never been attempted before, and it was decided this was a perfect opportunity to try it out.
PREFACE: the Al/Hg run in question was run as per Post 343969 (RoundBottom: "Os/BS AlHg MeAm Amination writeup", Methods Discourse), and at least 50g was expected from this particular attempt. upon final drying of toluene, the volume of toluene was measured (about 650mL), and split into 2 equal halves.
the first half was gassed immediately, as per usual, and a disappointing 16.1g was recovered where ~25g was expected. the toluene was gassed and vacuum filtered 6x, until, on the 6th gassing, there was no more precipitate. the standard H2SO4 on HCl generator with inline drying tube (filled with indicating dri-rite) was used.
the second half was left overnight and attended to the next afternoon. a 5% HCl solution was made (using muriatic acid, 31.45%), and slowly dripped into the beaker containing the toluene while the toluene was stirring on HIGH. the pH was checked after every eyedropper squirt (~1mL of 5% HCl). after many, many, many! squirts (got bored, lost count after about 30) the pH measured 3.5/4. the contents of the beaker were placed in a 500mL sep funnel, and allowed to separate. the bottom, aqueous layer (which had turned dark blue, possibly from the pH strip indicator?) was drained into a clean pyrex dish (the toluene was kept to make sure nothing was lost) and left to slowly evaporate the H2O. after a couple days the water had (mostly) evaporated, and a large, flat crystal sheet was on the bottom of the dish. it still had a dark blueish hue. this was scraped off, broken up and put in boiling acetone for 20 minutes, then filtered again. after drying, 25.3g clean salt was recovered.
even for a first attempt, titration seems to have won a new convert. a difference of almost 10g was achieved, and a whole lot of recovered toluene is going to be titrated for hidden remainders.
gassing stinks, is tedious, dirty, and can be dangerous. it also requires anhydrous conditions, which, if not achieved, may scuttle your yields. gassing's main advantage is it is quick.
titration has the advantage of doing away with anhydrous conditions, but it takes a while to evaporate the water and get crystals.
if you have the opportunity, give this experiment a try. the results may surprise you.
next up, sassy distillation vs. crystalization.
Now with 12% more Bottom!
Nymphomania is not a disease, its a goal! (Methadist)
(Stoni's sexual toy)
12-03-02 05:29
No 385796
Your results with gassing would have been much better if you had used less toluene, evaporated the toluene after the gassing and collected/processed the leftovers.
You also have to consider that your titrated product isn't anhydrous like the gassed product, it contains water of crystallisation. 100mg of each contain different amounts of active ingredient.
I'm not fat just horizontally disproportionate.
(Hive Addict)
12-03-02 13:10
No 385883
> gassing would have been much better if you had used less toluene
interesting. many have wondered what the proper amount of toluene is to extract freebase. and it is recommended to then reduce the volume of toluene to facilitate gassing? does this concentrate the freebase making it easier to receive the HCl?
> titrated product isn't anhydrous like the gassed product, it contains water of crystallisation
is this true after boiling briefly in acetone, filtering, and drying again? after doing this, the product has the same fluffy appearance as gassed product.
thanks for your insight, mr o.
Now with 12% more Bottom!
Nymphomania is not a disease, its a goal! (Methadist)
(Hive Bee)
12-03-02 13:45
No 385890
Cool RB!,
Swim has tried gassing and it works OK, but yields arent as good as one might like. Also setting up for gassing is tedious. I will also try your titration method!
Sink or SWIM
(Stoni's sexual toy)
12-03-02 14:40
No 385906
Great, here we go again, people found another way to do stuff 'the easy way'.
I suggest also skipping the A/B at the end to save yourselves some hassle and an hour or two of time.
Does that remind anyone of the quick and dirty 15 minute rP/I2 meth synth? It sure does to me.
I'm not fat just horizontally disproportionate.
(Hive Addict)
12-03-02 14:53
No 385914
i do not understand, is titration bad? i'm just excited that it's higher yielding. a rotovap is high on my list of TO GET.
is it possible to remove the h2o from the titrated crystals?
...and i've never skipped an A/B... i've never done one
Now with 12% more Bottom!
Nymphomania is not a disease, its a goal! (Methadist)
(Stoni's sexual toy)
12-03-02 15:00
No 385918
> i do not understand, is titration bad?
Hell no, isn't it common knowledge that product quality decreases the more you work on purity?
> is it possible to remove the h2o from the titrated crystals?
Sure it is, but why would you? Yields would suffer!
> ...and i've never skipped an A/B... i've never done one
I know. Otherwise you would have ended up with much less toluene. But who wants quality anway, I'm sure you could even produce pills by drying and compressing the whole Al sludge with the freebase trapped inside.
I'm not fat just horizontally disproportionate.
(Hive Bee)
12-03-02 15:09
No 385921
HAHA funny sarcasm. SWIM trys to get as pure product but still maintain good yields. Distilling the freebase would be even better huh!
Sink or SWIM
(Chief Bee)
12-04-02 02:37
No 386136
Have you ever thought of the fact that the reason you get "less yield" when distilling your freebase and recrystallizing your final product is due to you removing impurities from your product, and that you will be left with only MDMA.HCl (which of course weighs less than MDMA.HCl + impurities)?