05-22-03 18:00
No 434838
In Total Synsthesis II, starting on page 53, Strike tells us of a recurring dream of ketone synthesis. Questions: 1) The graphic shows the reaction starting with Safrole, the text says start with isosafrol. Which is correct? 2) After addition of the acetone solution, is the ice bath maintained overnight, or is no more ice required after the addition? Is it a matter of keeping the temp at 10 - 20 C overnight or just during the addition / reaction period? The motor in a stir plate could produce enough heat to cause the solution to exceed 20 degrees, but one could (in most areas) construct a flow-through waterbath using tapwater to keep the temp below 20 for extended periods.
(Hive Addict)
05-22-03 19:13
No 434853
that variation is very outdated and painful, read the performic oxidation on Rhodiums site. It uses dcm instead of acetone and the entire reaction is conducted at reflux, 40C, there are several work-up improvements you will like too. this rxn uses isosafrole.
you will need a condenser tho
(Chief Bee)
05-22-03 20:00
No 434870
Step 1: ../rhodium /isomeri
Step 2: ../rhodium /peracid
(Stranger)
05-30-03 17:30
No 436673
Thanks to Antibody2 and to Rhodium for the responses.
Poser: In Rhodium's "The Isomerization of Safrole: A Review" there is the statement that cis- and trans- isomers react the same, and there is no point in separating the two. Yet Strike's Total Synthesis II makes the contradictory statement that "the drug that will be made from it <cis-isomer> will not be recognized in the same way as trans in the brain cells of users."
Questions: Is cis- merely "double-jointed" trans? Is the distinction meaningless in the current context? I ask because Rhodium gives what could be a most convenient way to isolate cis- from trans- in the form of a table in the above-referenced work which includes the melting points of the cis- and trans- isomers. How convenient that most refrigerators (or an ice bath, for that matter) fall between the two melting points. Then you only have to hose off (ok, ok, vacuum filter) the cis- from the trans (or is this too simplistic)?
The Big Question: Is isolating the two isomers going to make a difference in the end product? Is is worth the (albeit small) effort?
Thanks to all in the hive who share the sacred knowledge, particularly to those gurus who know reaction mechanisms thoroughly enough to find new and novel methods; to those who keep an eye on the periodicals for pertinent break-throughs; and most particularly to those who have the patience to "dumb down" the info for those of us who don't have higher degrees in chemistry.
(Hive Bee)
05-30-03 18:05
No 436681
Is isolating the two isomers going to make a difference in the end product?
No. The information stated in the safrole isomerization review is correct.
fear fear hate hate
(Hive Bee)
05-30-03 19:15
No 436686
To most of us, I'm sure there is no difference. Not worth trying to seperate the isomers.
But I'm sure if we did kinetic studies with pure cis and pure trans we would get different speeds maybe even yields.
The oxidizing agent needs to coordinate with the isosafrole molecule or safrole(using Pd in a WAcker) to transform it into a ketone. I would tend to think the cis isomer would be oxidized more easily because the methyl and benzodioxole ring are cis to each other and allow the approach of the oxidizing agent. In the trans position, the pi bond would be more crowded, hindering more the reaction.
I know its only a methyl group I'm sure it slows down the oxidation.
But for our purposes, it doesnt really matter(at least not to me)
(Hive Bee)
05-30-03 20:59
No 436709
Agreed.
fear fear hate hate
(Newbee)
05-31-03 19:13
No 436893
isosafrole bought from aldrich contains both the cis and trans isomer... they do have differing boiling points.. so i supose you could seperate them...amine steriochemistry will undoubtly be relivant for biological activity... theres an interesting phd topic for someone there i think.
(Chief Bee)
06-01-03 14:50
No 437058
(Rated as: excellent)
The isomers cis and trans-isosafrole are simply isosafrole where the ring and the methyl group on the different sides of the double bond are either pointing in the same or in the opposite directions:
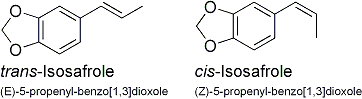
Each one will react in any oxidation to form the same end product as the other, both in the Wacker oxidation as well as in the peracid oxidation followed by the pinacol rearrangement, namely MDP2P.
The only thing that differs between the cis and trans isomers is the reaction rate, one may react a few times faster than the other, but will in time still produce the same thing. The statement made in Total Synthesis that they will give rise to different stereoisomers of the final amine is incorrect and chemically impossible, as the intermediate MDP2P is achiral.
(Stoni's sexual toy)
06-03-03 08:05
No 437543
Even if you start with pure cis or trans both isomers will temporarily exist when doing a wacker.
I'm not fat just horizontally disproportionate.
http://www.whatreallyhappened.com
(Stranger)
06-09-03 16:15
No 438927
Rhodium, I applaud your reviews for safrole isomerization and for peracid oxidation. Can I complete the series with reviews to various end products (majoring in MDMA, with a minor in MDA)?
By the way, your value as a resource is 10 to the nth power.
Thanks for being.