06-27-03 17:12
No 443020
Hellman Makes Methylamine in Vast quantities
Place equal amounts in weight of urea and GAA in a 2 necked round bottom flask, with air condensor. Heat gently to reflux, push back and crap that sublimes in condensor, this is all good, slowly bring the heat up to 195-200°C, this can take a while, say 1-1.5 h. When it gets to this point, stop the heating and let slowly cool. Now set up for distillation, heat to 200°C again, and you will find that between say 200-215°C (I found it roughly 210°C) acetamide collects in the reciever flask, upon cooling it solidifies into a big chunk. This is extremely pure Acetamide, don't get me wrong, there are no side-products.
Now take 125 g of acetamide in a 2 litre RBF and add 345g bromine (easily available) very fucking slowly - Danger! - to yield a deep red solution, just as the text book says. Cool the flask in an ice water bath, and add a 10% NaOH solution (approx 800ml) in small portions, with vigourous shaking until the solution takes on a pale yellow colour. This is essentially where bromoacetamide is present in the alkaline solution. If you see any solids crystallize, add enough water for them to re-dissolve.
Set up for distillation, but rather than using a reciever adapter, connect flexible tubing to the end of the condensor, and submerge the open end it in 500ml dilute HCl in a 1000 mL beaker, which the evolved gas is lead into. Kinda like a HCl generator, this is so that the produced gaseous methylamine freebase can form an aqueous solution of its hydrochloride salt upon exiting the distillation apparatus.
Into a dropping funnel place the above bromoacetamide solution, and into the RBF place 300g NaOH dissolved in 750 mL dH2O. Heat the RBF on a water bath to 60-70°C. Slowly drip the Bromoacetamide solution into the NaOH solution in the distillation flask, never letting the RBF solution to get above 75°C, If it does - just apply external cooling.
After all the solution has been added, maintain the solution in the RBF at 65-70°C for another 10 min, your indicator here to know when to stop warming is when the solution becomes clear. Drive of all the MeNH2 by gently warming the RBF. Evaporate off the HCl/MeNH2.HCl solution to give roughly 120g of crude MeNH2.HCl, the by-product being ammonium chloride. A nice boil with EtOH will remove all that, recrystallize, and dry the product in a desiccator over anhydrous CaCl2 to give Pure MeNH2.HCl.
Thank you again.
Ref: Vogel's Practical Organic Chemistry, 5th Ed. pp. 246-248.
IQ is the rate of logic, as Wisdom increases logic, fear & compromise decrease to reveal objectivity
(Hive Bee)
06-27-03 21:23
No 443047
Thank you for your contribution, I just wanted to acknowledge your post not just gain from reading it without letting you know this.. java
P.S. I also read your other stuff on the subject, it has come a long way from the two step method then..
Post 108611 (dormouse: "methylamine via glycine(Amino acid) (Page 1) -hellman", Novel Discourse)
We're all in this world together,
http://www.chiapaslink.ukgateway.net/
(Chief Bee)
06-28-03 14:27
No 443163
Hellman: I took myself the liberty of editing your post to improve grammar/spelling/sentence structure for clarity, as I believe it's a great post, and it would be a shame if it was missed by other people just because it was somewhat hard to read.
I'd like to add that 345 grams of elemental bromine is equal to 111 mL (Br2 density: 3.11 g/mL), it is much more convenient to quickly measure out a certain volume of bromine (the bottle advantageously cooled as much as possible before its opened, but not below -5°C or it solidifies) than dribble with it on your expensive balance before adding it to the addition funnel. Bromina vapors are nasty, and remember to use rubber gloves - bromine woulds heal very slowly!
However, I have a few questions regarding the work-up:
After all the solution has been added, maintain the solution in the RBF at 65-70°C for another 10 min, your indicator here to know when to stop warming is when the solution becomes clear. Drive of all the MeNH2 by gently warming the RBF.
Is the part where you drive away the last of the MeNH2 gas included in the above 10 minutes, or is it 10 min plus some more, and if so, is that also done at 65-70°C?
the by-product being ammonium chloride. A nice boil with EtOH will remove all that, recrystallize, and dry the product in a desiccator over anhydrous CaCl2 to give Pure MeNH2.HCl.
Ammonium chloride is no by-product of the Hofmann reaction betwen acetamide/bromine/NaOH - if there indeed is any ammonium chloride in the crude MeNH2.HCl, then that must come from ammonia or ammonium acetate contamination of the acetamide prepared in the first step.
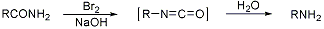
Above is the reaction mechanism for the rearrangement pictured, and here are links to some references on the Hofmann reaction (http://themerckindex.cambridgesoft.com/
Also, the purification must probably be clarified for many people - you separate the unwanted ammonium chloride by taking advantage of the fact that MeNH2.HCl is soluble in in boiling ethanol (denatured, I assume, considering the cost of pure 95% EtOH?), while the ammonium chloride is not - the full procedure being described in Organic Synthesis Coll. Vol. 1, p 347 (http://www.orgsyn.org/orgsyn/prep.asp?p
My last point is that your reference to Vogel's book is wrong, there is nothing about these reactions on page 246-248 in the 5th edition - are you thinking about the 3rd edition, or are your page numbers off?
(Soothsayer)
06-28-03 22:50
No 443221
--------Begin Ass Kissing--------
Rhodium,
Thankyou for your time and detailed response,
I will try to answer everything,.
Firstly, about the celubrious written recourse of my wicked mind,
I get carried away most of the time,with my writing, but it's kinda like altering a Picasso to make it layman friendly, wouldn't ya think?
But yes, this is chemistry, not Art; I like to blur the transitions a bit, sorry,.
I purposly let the grammer flow like that, I like to fuk with english, as it intrigues me,.
It's just what I do, I love words,.I know there are spelling mistakes,(I am a busy man, and haven't got time to always proof read)
Believe it or not I am a writer by passion, and have many years as a typesetter and proof reader, I think this is just a misunderstanding; but as this is for clarity for the bees, it is perfectly understandable,.
I do type way to fast,around 80 wpm, and don't give a shit enough to care essentially what people think, whether it's an arrogance thing,.I just try to capture the moment, and not write to impress.
I don't need people to think that I'm good, I already know it, Damn this Higher conciousness!!!!,.
How would you feel Rhodium if you were born with an IQ of 142?- you see there I go again,-Just playing,.
But the grammer and sentance structure are purpose built,.Please believe me, Please?........
I realize you Euro dudes have a thing for English, and that is exceptionally satifisfying, it is no lie that you Euro freaks can speak and write better than most of us English background plebians,.
Did you know that eye am from Australia, I reckon I'sa dooin pererty sweet, but?
I like the way I write, and like to get responses, especially from you,.
I hope you enjoyed also the p-tosic post,. Please be kind there too, the grammer and structure is a work of art,.
I hope you don't think that i am offended too much, just a little, I don't take critizism very well, you should know this by now,.
Maybee you could make it up to me by forwarding a few more of my posts to your page,.-smile
To answer your question, from Vogels 3rd Edition(Whoops)
Once you have added all the Bromoacetamide solution, maintain the solution at 65-70c for an additional 10 minutes, or as long as it takes to get the solution clear, either or, really, in my experience,.But to be safe I found that even once the solution went clear and colourless, I would still hold it there for an additional 10 minutes,.
Then you heat to drive of the Gas,.
Where does the Ammonium Chloride come from??
Very good question, Mr Vogel isn't sure either, where does it come from?, he doesn't really say, except for his comment on top of page 247, where he says that the reaction mechanisms are not completely understood in reference to the Hoffman,.
He says and I Quote" The mechanism of the reaction probably involve the following stages:"
That one word "probably" is where it is formed, later he also mentions the word presumerably,.
It's not absolute I know, This hurts me as well, I thought we could be more definate.
You say it comes from the acetamide,. I think I understand what you are saying, as the reaction mechamism of the Acetamide forms also carbamic acid which decomposes into Nh2, consequently being one of the byproducts, but that is taken care of by evapouration, and why would Mr Vogel start the Methylamine reaction with impure Acetamide,?
But the impurity(AmmCl2) is like 20% of the product in the MeNh2 generation, so there is a lot there, like 20g, if you do it to the scale I do,.
I think it must come from one of the 3 steps of the Hoffman reaction,.
either the
a) the formation of the N-haloamide or
b) In the presence of alkali, hydrogen halide is eliminated producing "presumably" an electronically-deficient nitrogen fragment (an acryl 'nitrene') which reaarranges to the isocyanate, or
c) Hydrolysis of the isocyanate to the carbamic acid , which is unstable and decomposes to the primary amine and caron dioxide.
It's is an interesting point, I am not an educated enough chemist, to see exactly where this might happen, but I think if Vogel was stuck, then I would have to say, it's a job for someone with a more thorough understanding,.(hint)
In retrospect I have a hunch,.
If the carbamic acid decomposes to Nh3 and Co2 in the acetamide Prep
Ch3COOH + NH2CONH2 -----------> CH3CONH2 + NH2COOH (--->CO2 + NH2)
then in the hoffman, carbamic is produced as well, so I would assume the Nh2 is made in situ, thus giving us a Nh2 as the byproduct,.
Blow me down, could that be correct, just maybee this reacts in Situ with the Hcl, Just a guess,
Goddamn I impress myself,.
Cheers
Hellmanus
IQ is the rate of logic, as Wisdom increases logic, fear & compromise decrease to reveal objectivity
(Hive Addict)
06-29-03 08:40
No 443300
Hey,
I dont see it on pg 247, your ref, in Vogels Practical Organic Chemistry, 3rd Edition.
(Hive Bee)
07-08-03 09:06
No 445612
A question though, from a non-chemist: could another Halogen (I'm thinking Chlorine or Iodine) be used instead of Bromine in this reaction?
(Chief Bee)
07-09-03 07:01
No 445861
The corresponding reaction with chlorine is known, but gives lower yields. I have not seen iodine or hypoiodite used.