(Chief Bee)
07-27-03 22:54
No 450229
(Rated as: excellent)
Catalytic Reduction of Nitriles and Oximes
Walter H. Hartung
J. Am. Chem. Soc. Vol. 50, 3370-3374 (1928)
In a study undertaken in this Laboratory it became desirable to reduce oximes and nitriles catalytically to pure primary amines. A review of the available literature showed that a large amount of research has been done on this type of reduction but that invariably a mixture of primary and secondary bases was obtained, with the latter often predominating.
Benzonitrile and benzaldoxime when reduced by gaseous hydrogen with palladium or nickel catalysts gave mixtures of benzylamine, dibenzylamine and ammonia.1 From
 - and
- and 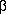 -naphthonitriles, phenylacetonitrile and
-naphthonitriles, phenylacetonitrile and 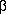 -phenylpropionitrile, Rupe and his coworkers obtained mixtures of the corresponding primary and secondary amines with ammonia. From acetaldoxime and propionaldoxime triethyl- and tripropylamines, respectively, and ammonia were obtained, while from benzophenone-oxime Paal and Gerum isolated a 57% yield of primary amine. Mignonac2 obtained ketimines from the oximes of cyclohexanone, acetophenone and propiophenone. Braun, Blessing and Zobel3, by reducing nitriles in various non-aqueous solvents, found that they obtained both primary and secondary amines, the amount of each depending on the concentration and on the nature of the solvent. Vavon and Berton4 report that cyclic ketoximes reduced with platinum from hydroxylamines.
-phenylpropionitrile, Rupe and his coworkers obtained mixtures of the corresponding primary and secondary amines with ammonia. From acetaldoxime and propionaldoxime triethyl- and tripropylamines, respectively, and ammonia were obtained, while from benzophenone-oxime Paal and Gerum isolated a 57% yield of primary amine. Mignonac2 obtained ketimines from the oximes of cyclohexanone, acetophenone and propiophenone. Braun, Blessing and Zobel3, by reducing nitriles in various non-aqueous solvents, found that they obtained both primary and secondary amines, the amount of each depending on the concentration and on the nature of the solvent. Vavon and Berton4 report that cyclic ketoximes reduced with platinum from hydroxylamines.Rosenmund and Pfankuch5 were the first to forestall the formation of secondary amine by using the acetate of the oxime and an acetic acid solution of the nitrile. Thus benzaldoxime acetate gave a 91% yield and benzonitrile an 80% yield of benzylamine, benzylcyanide gave a 73% yield of phenylethylamine, while p-hydroxybenzylcyanide again formed secondary amine.
Hence it appears that except for the methods of Rosenmund and Pfankuch and Carothers and Jones,6 there is no known general procedure for the catalytic hydrogenation or reduction of nitriles and oximes to the corresponding pure primary amines.
An investigation in this Laboratory shows that it is possible to reduce benzonitrile and benzaldoxime to primary amine without contamination from the secondary base, by carrying out the reduction with palladinized charcoal in a solution of absolute alcohol containing hydrogen chloride, the base being isolated as the salt. Benzonitrile with one equivalent, or more, of hydrogen chloride was reduced very smoothly to benzylamine hydrochloride. Benzaldoxime yielded similar results when three equivalents of hydrogen chloride were used, whereas with one equivalent a mixture of the salts of the primary and secondary bases was obtained. When no acid was present both the nitrile and oxime formed benzylamine, dibenzylamine and ammonia, as previous investigators have found.
Mandelonitrile was also reduced under similar conditions. The product, however, was not phenylethanolamine, as one might expect, but phenylethylamine, for the alcoholic hydroxyl was also reduced; this happened even when the reaction was stopped on the absorption of sufficient hydrogen to reduce theoretically only the nitrile group; but the yield of primary amine salt was very low, 52% of the theoretical, and a nonbasic byproduct was formed which was not identified. Much better yields of phenylethylamine hydrochloride were obtained by starting with the benzoic and acetic acid esters of mandelonitrile. Esterification of the hydroxyl group did not protect it, for both the benzoate and acetate resulted in good yields of phenylethylamine hydrochloride. A similar reduction of the hydroxyl group, also catalytically, is recorded by Rosenmund and Schindler7, who obtained phenylethylacetic acid and substituted phenylethylacetic acids from mandelic and substituted mandelic acids where the hydroxyl group had been esterified.
An attempt to reduce benzonitrile in a solution of dibutyl ether without hydrogen chloride was a failure for only about a tenth of the hydrogen calculated for the complete reduction was absorbed. That the catalyst had not become poisoned was demonstrated by the fact that it subsequently reduced benzonitrile in absolute alcoholic solution. An attempt to reduce mandelonitrile acetate in benzene solution also resulted in failure.
Procedure and Apparatus
The reduction was carried out by shaking the substance to be reduced in an atmosphere of hydrogen delivered from a graduated cylinder under, a pressure of approximately 30 in. of water.
The oxime or nitrile was dissolved in the proper amount of absolute alcohol and placed along with the catalyst in the reaction flask. The reaction flask was connected to the hydrogen delivery train by means of a ground-glass joint held together with rubber bands caught over small projecting glass hooks. The only rubber connection throughout the whole apparatus was that joining the hydrogen tank to the delivery tube. The air was removed by evacuating the apparatus, filling with hydrogen and repeating the process at least four times, which left an atmosphere of practically pure hydrogen. No hydrogen was taken up until agitation was begun.
Reagents and Materials
The catalyst was palladinized charcoal, prepared according to the directions of Ott and Schröter8. Three grams of pure animal charcoal from Merck and Co. was shaken with an aqueous solution of 0.45-0.50 g. of palladium chloride in an atmosphere of hydrogen until saturated. The charcoal was then filtered off, washed, dried and kept in vacuo over sulfuric acid until used. Each catalyst could be used for three or four experiments.
The hydrogen was a pure, commercial, electrolytic product. Before reaching the reduction flask it was passed through an alkaline solution of pyrogallol, a solution of sodium hydrosulfite and then through a long tube containing calcium chloride and soda lime. The hydrogen so purified was entirely satisfactory and good results were obtained with it.
The benzonitrile was used as obtained from Eastman Kodak Company. The benzaldoxime was prepared from benzaldehyde and hydroxylamine and boiled at 101-103°C at 6-7 mmHg.9
The mandelonitrile was prepared according to the directions of Ultêe.10 To a mixture of 26.5 g. (0.25 mole) of freshly distilled benzaldehyde and 8.2 g. (0.3 mole) of hydrogen cyanide11 was added a few drops of concentrated potassium hydroxide solution as catalyst. The temperature rose spontaneously to 50°C; after it began to cool, the mixture was placed in a freezing bath and within about an hour, with occasional shaking, crystals began to appear on scratching the walls of the container, and very soon almost the whole mixture was solid. The crystals were removed by suction on a previously cooled Buchner funnel and by again freezing the filtrate more crystals were obtained. The crystalline product was pressed, in the ice-box, between two porous plates to remove non-crystallizable matter; this left a beautiful white product that melted at 20°C, which agrees with the figure given by Ultêe. "International Critical Tables," Vol. I, gives the melting point for mandelonitrile as -15°C. No medium was found for recrystallizing the mandelonitrile, not even when the solutions were chilled in a mixture of ether and carbon dioxide snow.
Mandelonitrile benzoate was prepared as described by Francis and Davis.12 Mandelonitrile acetate was prepared according to the directions of Michael and Jeanprêtre.13
Experimental Results
Reduction of Benzaldoxime.
Three grams of benzaldoxime (0.025 mole) was dissolved in 40 mL of absolute alcohol containing 3 equivalents of hydrogen chloride, and in ninety minutes reduction was complete. The catalyst was removed by filtration, the first aqueous washing evaporated to dryness, the residue taken up in hot absolute alcohol and added to the filtrate. The alcoholic filtrate was partially evaporated and diluted with excess ether. The crystals thus obtained melted at 258°C; the yield of hydrochloride was practically quantitative. When but one equivalent of hydrogen chloride was used the product melted at 220-230°C; it formed two benzenesulfonyl derivatives, the alkaline insoluble one melting at 67°C14 and the alkaline soluble derivative melting at 86-87°C.15 The primary amine predominated, however. When no hydrogen chloride at all was used, a mixture of primary and secondary bases resulted.
Benzonitrile reduced under the same conditions as described for benzaldoxime resulted in pure benzylamine hydrochloride. Here, however, one equivalent of hydrogen chloride, or more, prevented the formation of secondary amine, whereas without hydrogen chloride a mixture of benzylamine, dibenzylamine and ammonia was obtained.
Reduction of Mandelonitrile.
A solution of 5.8 g. of mandelonitrile (0.0436 mole) in 90 mL of absolute alcohol containing 0.049 mole of hydrogen chloride absorbed 2080 mL. of hydrogen in ninety minutes; the amount calculated for reduction to phenylethanolamine was 1970 mL; for complete reduction to phenylethylamine, 2950 mL. The catalyst was removed by filtration and the filtrate was reduced to a volume of about 15 mL and diluted with excess ether, which caused a crystalline solid to settle out; the solid, after recrystallization from absolute alcohol, melted at 215°C.16 The free base distilled at 200°C17 and when exposed to the air quickly formed a solid which effervesced with dilute acid and after washing with ether melted at 103°C.18 The product formed an alkali-soluble benzenesulfonyl derivative which melted at 68-69°C.19
It formed a benzoyl derivative which melted at 120°C.20 Comparison of the product here obtained and its derivatives with known phenylethanolamine hydrochloride and its corresponding derivatives, showed them to be distinctly different compounds. Thus mandelonitrile was reduced to phenylethylamine. The salt is, however, obtained in small yield (52%) for rectification of the alcohol-ether mother liquors showed that a non-basic by-product was formed which was not identified.
Reduction of Mandelonitrile Benzoate.
A solution of 5.9 g. (0.025 mole) of mandelonitrile benzoate in 60 mL. of absolute alcohol containing 0.057 mole of hydrogen chloride absorbed, in sixty minutes, 1640 mL of hydrogen; calculated for reduction to the benzoate of phenylethanolamine, 1125 mL; for reduction to phenylethanolamine, 1680 mL The product, isolated in the usual manner, gave the characteristic tests for phenylethylamine hydrochloride and was obtained in 84.5% yield. Attempts to use only one equivalent of hydrogen chloride, added during the course of reduction, also resulted in phenylethylamine but in poorer yields and with contaminating by-products.
The reduction of mandelonitrile acetate was carried out after the manner of the benzoate. From 4.4 g. (0.025 mole) of the nitrile acetate there was obtained in 75 min 3.9g of phenylethylamine hydrochloride, a yield of 74.5%.
Conclusions
[1] Benzonitrile was reduced catalytically in absolute alcoholic solution to pure benzylamine by means of palladinized charcoal when one equivalent, or more, of hydrogen chloride was present; without hydrogen chloride a mixture of benzylamine, dibenzylamine and ammonia was formed.
[2] Benzaldoxime was similarly reduced to pure benzylamine when three equivalents, or more, of hydrogen chloride were present. With one equivalent, or more, of the acid a mixture of the primary and secondary bases resulted.
[3] Mandelonitrile was reduced in absolute alcoholic solution containing hydrogen chloride to phenylethylamine, but the yields of primary amine were poorer because a non-basic by-product was formed.
[4] The benzoate and acetate of mandelonitrile were also reduced; though the esters of phenylethanolamine were not formed, good yields of phenylethylamine were obtained.
References
[1] (a) Paal and Gerum, Ber., 42, 1553 (1908); (b) Rupe and Beckerer, Helv. Chim. Acta, 6, 880 (1923); (c) Rupe and Glenz, Helv. Chim. Acta, 5, 937 (1922); (d) Rupe and Hodel,Helv. Chim. Acta, 6, 865 (1923); (e) Gulewitsch, Ber., 57, 1645 (1924).
[2] Mignonac, Compt. rend., 170, 936 (1920).
[3] Braun, Blessing and Zobel, Ber., 56, 1988 (1923).
[4] Vavon and Berton, Bull. Soc. Chim., 37, 296 (1925).
[5] Rosenmund and Pfankuch, Ber., 56, 2258 (1923).
[6] Carothers and Jones, J. Am. Chem. Soc, 47, 3051 (1925), using platinum catalyst in acetic anhydride solvent, reduced several nitriles to the corresponding primary amines, isolating them as their acetyl derivatives, from which the free amine could be obtained by subsequent hydrolysis.
[7] Rosenmund and Schindler, Arch. Pharm., 266, 281 (1928).
[8] Ott and Schröter, Ber., 60, 633 (1927).
[9] Bourgeois and Dambmann, Ber., 26, 2857 (1893).
[10] Ultêe, Rec. Trav. Chim., 28, 254 (1909).
[11] Ziegler, "Organic Syntheses," John Wiley and Sons, Inc., New York, 7, 50 (1927).
[12] Francis and Davis, J. Chem. Soc., 95, 1404 (1909).
[13] Michael and Jeanprêtre, Ber., 25, 1681 (1892).
[14] Mp of benzenesulfonamide derivative of dibenzylamine 68°C [Beckmann and Pellrath, Ann., 273, 22 (1893)].
[15] Mp of N-benzylbenzenesulfonamide, 88°C [Hinberg, Ann., 265, 182 (1891) ].
[16] Mp of phenylethylamine hydrochloride 217°C (Mulliken, "Identification of Pure Organic Compounds," John Wiley and Sons, Inc., New York, 1916, Vol. II, p. 146.)
[17] Bp of phenylethylamine, 197-198°C/725mmHg. (Mulliken16).
[18] Phenylethylamine carbonate melts at 101-104°C [Pileti and Piccini, Ber., 12, 1700 (1879)].
[19] Johnson and Guest, Am. Chem. J., 42, 248 (1909), give the mp of N-phenylethylbenzenesulfonamide as 68-69°C.
[20] Mp of N-phenylethylbenzamide, 113-114°C [Bischler and Napieralski, Ber., 26, 1905 (1893)].
(Hive Addict)
07-28-03 14:42
No 450370
(Rated as: excellent)
Some references can be consulted online for free.
G Mignonac. CR 170 (1920) 936 (http://gallica.bnf.fr/scripts/get_page.
H Schiff. Ber 12 (1879) 1698 (http://gallica.bnf.fr/scripts/get_page.
H A Michaël and J Jeanprêtre. Ber 25 (1892) 1678 (http://gallica.bnf.fr/scripts/get_page.
A Bischler. Ber 26 (1893) 1891 (http://gallica.bnf.fr/scripts/get_page.
E Bourgeois and J Dambmann. Ber 26 (1893) 2856 (http://gallica.bnf.fr/scripts/get_page.
Edit: the PDFs you will download by clicking the link is always the 1st page of the article. By being a little bit creative - for instance by increasing the number in the link by 1 - you can view the following pages as well. Or you can go directly to the BNF (http://gallica.bnf.fr) website.
Dirty old man