(Chief Bee)
08-21-03 02:54
No 454765
(Rated as: excellent)
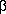 -3,4-Methylenedioxyphenylisopropylamine
-3,4-MethylenedioxyphenylisopropylamineJ. Elks and D. H. Hey
J. Chem. Soc, 15-16 (1943)
Abstract
A method is described for the preparation of
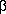 -3,4-methylenedioxyphenylisopropylamine from piperonal, which may be regarded as a prototype for a general method for the preparation of substituted
-3,4-methylenedioxyphenylisopropylamine from piperonal, which may be regarded as a prototype for a general method for the preparation of substituted 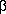 -phenylisopropylamines from aromatic aldehydes.
-phenylisopropylamines from aromatic aldehydes.Symphatomimetic amines related to amphetamine are conveniently prepared from the appropriate ketone, usually by the reduction of the oxime. Amphetamine itself is obtained in this way by the reduction of benzyl methyl ketoxime (Hey, J. Chem. Soc. 18, 1930). The preparation of similar bases with substituent groups in the aromatic nucleus is dependent on the accessibility of the corresponding substituted benzyl methyl ketones. Such ketones may be conveniently prepared by the method of Darzens (Compt. Rend., 1906, 142, 214), which consists in the condensation of an aromatic aldehyde with an
 -halogeno-aliphatic ester in presence of sodium ethoxide, followed by hydrolysis and decarboxylation of the resulting glycide ester, thus:
-halogeno-aliphatic ester in presence of sodium ethoxide, followed by hydrolysis and decarboxylation of the resulting glycide ester, thus: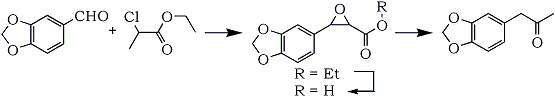
In view of the recent publication of Patent GB519894 which directs attention to this route to substituted
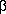 -phenylisopropylamines from the corresponding substituted benzaldehydes, it is desired to place on record work on these lines commenced in 1939 but interrupted owing to the war. This work was undertaken with the object of developing this series of reactions as a general method for the preparation of substituted
-phenylisopropylamines from the corresponding substituted benzaldehydes, it is desired to place on record work on these lines commenced in 1939 but interrupted owing to the war. This work was undertaken with the object of developing this series of reactions as a general method for the preparation of substituted 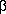 -phenylisopropylamines, many of which are already known to possess physiological activity, of interest. The only synthesis completed was that of
-phenylisopropylamines, many of which are already known to possess physiological activity, of interest. The only synthesis completed was that of 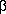 -3,4-methylenedioxyphenylisopropylamine and details of this preparation given below may serve as a prototype for the general method.
-3,4-methylenedioxyphenylisopropylamine and details of this preparation given below may serve as a prototype for the general method.The condensation of piperonal with ethyl
 -bromopropionate gave
-bromopropionate gave 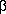 -3,4-methylenedioxyphenyl-
-3,4-methylenedioxyphenyl- -methylglycidic ester, which on subsequent hydrolysis and decarboxylation gave 3,4-methylenedioxybenzyl methyl ketone. Finally
-methylglycidic ester, which on subsequent hydrolysis and decarboxylation gave 3,4-methylenedioxybenzyl methyl ketone. Finally 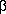 -3,4-methylenedioxyphenvlisopropylamine was prepared from the latter by means of the Leuckart reaction, i.e., heating the ketone with formamide and hydrolysing the resulting formyl derivative (c.f. Ingersoll. Brown. Kim, Beauchamp, and Jennings, J. Amer. Chem. Soc., 1936, 58, 1808).
-3,4-methylenedioxyphenvlisopropylamine was prepared from the latter by means of the Leuckart reaction, i.e., heating the ketone with formamide and hydrolysing the resulting formyl derivative (c.f. Ingersoll. Brown. Kim, Beauchamp, and Jennings, J. Amer. Chem. Soc., 1936, 58, 1808).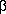 -3,4-Methylenedioxyphenylisopropylamine has been prepared by Mannich and Jacobsohn (Ber., 1910, 43, 194) by the reduction of the oxime of 3,4-methylenedioxybenzyl methyl ketone, which in turn was prepared by the action of heat on isosafrole oxide (Hoering, Ber., 1905, 38, 3481). Several workers have prepared 3,4-methylenedioxybenzyl methyl ketone from derivatives of isosafrole.
-3,4-Methylenedioxyphenylisopropylamine has been prepared by Mannich and Jacobsohn (Ber., 1910, 43, 194) by the reduction of the oxime of 3,4-methylenedioxybenzyl methyl ketone, which in turn was prepared by the action of heat on isosafrole oxide (Hoering, Ber., 1905, 38, 3481). Several workers have prepared 3,4-methylenedioxybenzyl methyl ketone from derivatives of isosafrole.Experimental
3,4-Methylenedioxybenzyl Methyl Ketone
Freshly prepared powdered sodium ethoxide (23 g) was added during 4 hours to a stirred mixture of piperonal (50 g) and ethyl
 -bromopropionate (61 g) cooled in ice-salt. Stirring was continued overnight at room temperature and for 6 hours on the water-bath; ice-water was then added, and the mixture acidified with dilute acetic acid. The glycide ester was extracted with ether, and the ethereal solution washed with aqueous sodium carbonate and dried with sodium sulphate. After removal of ether the residue was distilled and collected between 70-200°C/20 mmHg. Subsequent redistillation gave
-bromopropionate (61 g) cooled in ice-salt. Stirring was continued overnight at room temperature and for 6 hours on the water-bath; ice-water was then added, and the mixture acidified with dilute acetic acid. The glycide ester was extracted with ether, and the ethereal solution washed with aqueous sodium carbonate and dried with sodium sulphate. After removal of ether the residue was distilled and collected between 70-200°C/20 mmHg. Subsequent redistillation gave 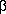 -3,4-methylenedioxyphenyl-
-3,4-methylenedioxyphenyl- -methylglycide ester (40 g), bp 184-186°C/14 mmHg, 48% yield. Darzens (loc. cit.) recorded bp 205-210°C/25 mmHg. The ester (35 g) was refluxed for 5 hours with a solution of sodium hydroxide (10 g) in 90% aqueous alcohol (150 mL). After removal of part of the alcohol by distillation the residue was diluted with a large volume of water and extracted with a small quantity of ether. The aqueous layer was acidified with dilute hydrochloric acid, the mixture extracted with ether, and the extract dried over sodium sulphate. The free acid obtained on removal of the ether was refluxed for 18 hours at 180°C with the addition of a trace of copper powder; the liquid was then distilled slowly under reduced pressure in the presence of copper powder. Re-distillation of the product gave 3,4-methylenedioxybenzyl methyl ketone (11.1 g), bp 154-156°C/11 mmHg, 44.5% yield. Mannich and Jacobsohn (loc. cit.) recorded bp 168°C/17 mmHg. and Hoering (loc. cit.) bp 149-151°C/10 mmHg. The semicarbazone, prepared in the usual manner, separated from aqueous alcohol in white needles, mp 161-162°C. Wallach and Müller (Annalen, 1904, 332, 333) recorded mp 163°C.
-methylglycide ester (40 g), bp 184-186°C/14 mmHg, 48% yield. Darzens (loc. cit.) recorded bp 205-210°C/25 mmHg. The ester (35 g) was refluxed for 5 hours with a solution of sodium hydroxide (10 g) in 90% aqueous alcohol (150 mL). After removal of part of the alcohol by distillation the residue was diluted with a large volume of water and extracted with a small quantity of ether. The aqueous layer was acidified with dilute hydrochloric acid, the mixture extracted with ether, and the extract dried over sodium sulphate. The free acid obtained on removal of the ether was refluxed for 18 hours at 180°C with the addition of a trace of copper powder; the liquid was then distilled slowly under reduced pressure in the presence of copper powder. Re-distillation of the product gave 3,4-methylenedioxybenzyl methyl ketone (11.1 g), bp 154-156°C/11 mmHg, 44.5% yield. Mannich and Jacobsohn (loc. cit.) recorded bp 168°C/17 mmHg. and Hoering (loc. cit.) bp 149-151°C/10 mmHg. The semicarbazone, prepared in the usual manner, separated from aqueous alcohol in white needles, mp 161-162°C. Wallach and Müller (Annalen, 1904, 332, 333) recorded mp 163°C.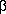 -3,4-Methylenedioxyphenylisopropylamine
-3,4-MethylenedioxyphenylisopropylamineAmmonium formate (14 g) was heated under distillation conditions to 160°C, the water which was collected being rejected; the ketone (9.9 g) was then added and the temperature maintained at 160-165°C. The ketone which distilled over was returned to the reaction flask from time to time. The heating was continued for 5 hours after the distillation of water ceased. The mixture was allowed to cool and was shaken with twice its volume of water. The formyl derivative of the amine was extracted with ether, the ether removed, and the residual oil hydrolysed by refluxing with hydrochloric acid (d 1.16, 8 mL) for 45 minutes. To the cooled mixture, more hydrochloric acid (2 mL) was added, and the whole extracted with ether. The aqueous layer was then made alkaline and again extracted with ether, the extract dried over potash, the ether removed, and the residue distilled under reduced pressure.
 -3,4-Methylenedioxyphenylisopropylamine (2.0 g) was collected at 138-140°C/12 mmHg, 20% yield. Mannich and Jacobsohn (loc. cit.) recorded bp 157°C/22 mmHg.
-3,4-Methylenedioxyphenylisopropylamine (2.0 g) was collected at 138-140°C/12 mmHg, 20% yield. Mannich and Jacobsohn (loc. cit.) recorded bp 157°C/22 mmHg. A portion of the amine (1 g) was warmed with acetic anhydride (1 g) on the steam-bath for 30 minutes. The liquid was poured into water, and the mixture boiled for a few minutes and cooled. The solid obtained melted at 59-61°C after crystallisation from aqueous alcohol and drying in air. After a second crystallisation from aqueous alcohol, followed by drying in a vacuum, the mp was raised to 93°C. Direct crystallisation from benzene-light petroleum also gave the acetyl derivative in white fibrous needles, mp 93°C. The acetyl derivative of amphetamine (Hey, loc. cit.) exhibits a similar behaviour.
Note: "loc. cit." = "See above citation (by that author)" = loco citato (http://www.bartleby.com/61/12/L0221200.
Reference links:
dl-beta-Phenylisopropylamine and Related Compounds
By Donald Holroyde Hey
J. Chem. Soc. 18 (1930) = Post 402025 (Rhodium: "Ye olde Benzedrine", Stimulants)
Extensions of the Leuckart Synthesis of Amines
A. W. Ingersoll, J. H. Brown, C. K. Kim, W. D. Beauchamp, G. Jennings
J. Am. Chem. Soc. 58(9), 1808-1811 (1936) (../rhodium/pdf /leuckart.ami
(Chief Bee)
07-03-04 17:26
No 517181
Über Oxyphenyl-alkylamine und Dioxyphenyl-alkylamine
C. Mannich & W. Jacobsohn
Chem. Ber. 43, 189-197 (1910) (../rhodium/pdf /mda.synthesi
The Hive - Clandestine Chemists Without Borders