04-27-04 21:51
No 503363
i read someplace that sodium bisulfite is supposed to break vitamin b1 into two parts, one of which is a controlled substance (sedative). this is all i have. i don't even remember the name of the sedative, although i think the dose was 100mg. this was mentioned in pills a go-go (book) which names sedative compound and dose but chemical literature stated cleaving compound as sodium bisulfite, not compound stated in pills a go-go. can anyone confirm or deny foregoing?
(Chief Bee)
04-28-04 03:28
No 503399
Yes, Vitamin B-1 can be cleaved with bisulfite:
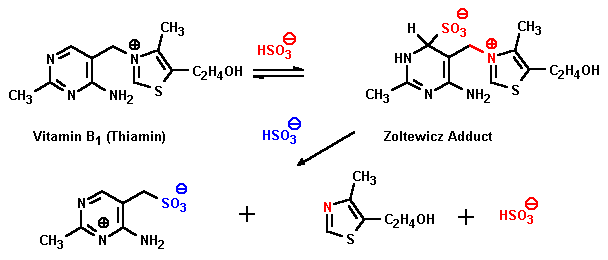
However, the alcohol formed - 2-(hydroxyethyl)-4-methyl-thiazole - needs to have its OH group substituted with a chlorine atom before the result becomes the anxiolytic/hypnotic Clomethiazole.
The Hive - Clandestine Chemists Without Borders
(Hive Bee)
06-25-04 06:11
No 515281
Rhodium, would gassing with Cl2 be sufficient for the chlorination? or must some other method bee used? this sounds like an interesting synthesis.
A politician is like a baby's nappy, it should be changed often and for the same reason.
(Hive Bee)
06-25-04 08:15
No 515295
Lestat: This is a nucleopylic substitution of the alcohol to the chlorine group - not the usual chloriantion with Cl2. You need reagents like SOCl2 or PCl3. Some alcohols can bee also substituted with conc. HCl but not the primary ones like this one.
“The real drug-problem is that we need more and better drugs.” – J. Ott
(Faux News)
06-26-04 21:03
No 515575
How about ZnCl2/HCl?
http://www.geocities.com/dritte123/PSPF.
(Über-Führer die Ironie)
10-10-04 20:35
No 535211
(Rated as: excellent)
Some alcohols can bee also substituted with conc. HCl but not the primary ones like this one.
Seems like HCl works under a bit harsh conditions:
Studies of Crystalline Vitamin B1. XIV. Sulfite Cleavage. IV. The Thiazole Half
Edwin R. Buchman;
J. Am. Chem. Soc.; 1936; 58(9); 1803-1805.

4-Methyl 5-(beta-Chloroethyl) Thiazole - Two grams of the hydroxythiazole was heated with 25 cc. of concentrated hydrochloric acid for three hours in a sealed tube at 145°C. After evaporation of excess acid, the free base was liberated with alkali and isolated by distillation; yield 1.5 g. The compound has a characteristic odor and is stable at room temperature: b. p. 74-75° (3 mm.)
And the cleavage of the Vitamine:
Studies of Crystalline Vitamin B1. III. Cleavage of Vitamin with Sulfite
Robert R. Williams, Robert E. Waterman, John C. Keresztesy, Edwin R. Buchman;
J. Am. Chem. Soc.; 1935; 57(3); 536-537.

1.000 gram of vitamin was dissolved in 15 cc. of sodium sulfite solution containing sufficient excess sulfurous acid to bring the pH to 4.8-5.0. The total sulfite content was 2.6 N. After standing overnight at room temperature the liquid had deposited copious amounts of the sparingly soluble acidic cleavage product in crystalline form. After standing for several days, the crystalline product was collected, washed and dried; weight 535.8 mg.
The mother liquor and washings were brought to pH 10 with strong sodium hydroxide and the alkaline solution extracted seven times with 50 cc. of chloroform each time.
The combined chloroform extracts were extracted with dilute hydrochloric acid, the acid aqueous extract was evaporated in vacuo and the residue was extracted with absolute alcohol. The alcoholic solution on evaporation left a residue of 518.8 mg. of the crystalline hydrochloride of the basic cleavage product, the purity of which was demonstrated by analysis; yield 97.40%,. The recrystallization of this material is effected by dissolving in a minimum amount of absolute alcohol, adding an excess of dioxane and allowing to stand.
From the alkaline liquor after chloroform extraction, a further quantity of 25 3 mg. of acidic cleavage product was obtained by neutralizing and concentrating to a small volume; total yield 561 mg. or 93.1%, of the theoretical. Fine white needles are obtained on recrystallization from hot water.
After some random web-browsing I understand that this is a GABA agonist which seems to have effects simillar to those of GHB and is neuroprotective too. If it's not a fun compound, maybee it should bee used by MDxA bees beefore and after sessions, like zolopht;
The activation of the GABAergic system might inhibit the release of dopamine leading to a reduction of dopamine-mediated neurotoxicity (reviewed in, Green et al., 1995). The administration of clomethiazole before and after MDMA exposure revealed a neuroprotective effect in neocortex and hippocampus (Colado et al., 1992).
Source: http://www.maps.org/w3pb/new/2002/2002_o
(maybee this thread shall bee moved?)
(Hive Bee)
10-11-04 20:27
No 535378
Ha! Now i remeber where i know Clomethiazole from:
In germany it's used for Alcohol withdrawal - in the old days when
swim was working in a hospital people with alcohol withdrawal or
even delire where treated with it i.v (per infusion) or oraly.
Someone (no alco fiend :))told me he was feeling pretty drunk after
a dose of distraneurin-pills. maybe it is really somehow like ghb?
- Beware of the Morphail Effect! -