04-19-00 11:24
No 126498
What is the approximate size of the largest distillation flask that I could use with a short-path condenser for the distillation of MDP2P & safrole?
(Moderator)
04-19-00 13:54
No 126499
Depends on how small that condenser thing is. I have used 500ml flasks with small scale distillation equipment, and it worked very well. You must be able to control the temp pretty good to avoid overheating and too violent boiling, this usually means an oil bath is necessary, don't use a heating mantle.
(Moderator)
06-19-04 04:08
No 514231
(Rated as: good read)
This design should prove useful to those needing a suitable design for a short path condenser
Synthetic Communications 26, 3421-4 (1996)
Distillation of less than a ml of sample can be accomplished with a jacketed Hickman still. The jacket allows positioning of a coolant such as dry ice/acetone at the site of desired condensation. A jacketed distillation head will accomodate samples up to a volume of 3 ml. Distillate is easily removed with a Pasteur pipette. Details of the glassware design are given.

Chemistry is our Covalent Bond
(Chief Bee)
06-19-04 21:00
No 514298
(Rated as: good read)
A Microscale Vacuum Distillation Apparatus for Simple Separations
Ulf Ellervik and Hans Grundberg
J. Chem. Ed. 76(7), 896 (1999)
In an ongoing project to adapt microscale techniques to our basic organic chemistry course we needed a simple, inexpensive, and highly efficient vacuum distillation apparatus. Several reactions give liquid products (usually in the 0.2–0.5 mL range) suitable for purification by distillation. Since common microscale equipment was rather inefficient and retained a large fraction (0.07–0.14 mL) of the material after distillation, we designed a new apparatus (Fig. 1). Several test runs in our equipment with diethylmalonate gave a good yield of distilled material and only a small amount remained (ca. 0.025 mL). The apparatus (Fig. 1) is made of a tube with an NS 10 joint that is bent twice and fused with a water-cooled condenser. The tube is then connected to a vacuum pump or water aspirator. The distillate is collected in a 1.75-mL glass vial that is tightened with an O-ring or a rubber gasket, simply made from a rubber sheet using a cork borer. The purity of the product is controlled by refractive index or a microscale boiling point determination. This apparatus has been used in our student laboratories with very good results. The distillation is fast and efficient and the equipment is very easy to handle.
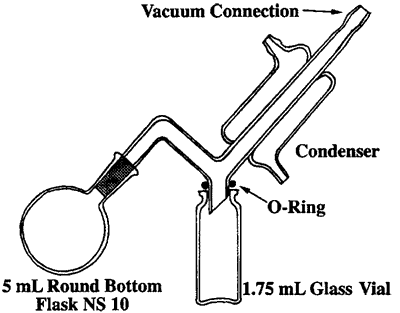
Figure 1.
Microscale vacuum distillation apparatus.
The Hive - Clandestine Chemists Without Borders