03-23-04 01:19
No 496725
SWID was wondering if anybody had any references other than the ones posted, or theories on the mechanistics of this reduction.
SWID is thinking the reaction occurs on a 1:1 molar basis, where one molecule of benzaldehyde plus one molecule of o-phenlyenediamine condese to form PBI + H2 + H2O. Seeing the production of the hydrogen (in H radical form?) shows where the hydrogen is coming from, but doesn't really explain how this is able to add across the double bond.
So questions are:
1. Mechanistics?
2. 91% IPA ok? (H2O shouldnt be a problem)
3. this only works if PBI is generated in situ? Since the PBI is isolated during workup, might it be possible to use it as a catalyst w/ hydrogen introduced from an outside source? (SWID is thinking not, be he is often thinking wrong, so).
4. Is inert atmosphere necessary? What about a hydrogen atmosphere?
Links, replies, comments, etc very much appreciated.
Drug Chemists are Ta to a good Sm.
(Chief Bee)
03-23-04 02:21
No 496740
When reacting benzaldehyde with phenylenediamine, you get 2-phenyl-benzimidazoline (PBI) through an intermediate imine with the loss of water. No hydrogen is lost.
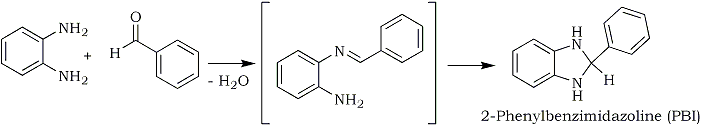
Upon reacting PBI with a hydrogen acceptor like a nitrostyrene (see Post 352950 (Barium: "Get that double bond without borohydride", Novel Discourse)), the products are the saturated nitroalkane and 2-phenyl-benzimidazole:
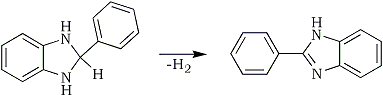
The driving force in the reduction is the formation of the aromatic heterocycle (resonance energy) from the aliphatic nitrogen ring in the benzimidazoline. I think the reaction proceeds through some kind of hydride transfer, so no free hydrogen (radical or molecular) is ever formed.
The Hive - Clandestine Chemists Without Borders
(Hive Bee)
03-23-04 02:53
No 496747
Wonderful! Thanks to the Chief Bee from SWID, this whole reaction makes a LOT more sense now. (SWID was gonna edit and ask why when the two reagents are added they don't release any gaseous hydrogen, now he knows!)
Drug Chemists are Ta to a good Sm.
(Hive Bee)
03-23-04 03:14
No 496753
To answer SWIDs own questions:
2. No prob, just a bit longer reflux since lower temp.
3. Cannot reuse the recovered imidozole. (Regeneration? Probably not worth it.)
4. Looks like the atmospheric danger is largely from oxygen, oxidizing the -oline to ozole.
Drug Chemists are Ta to a good Sm.