06-12-04 07:52
No 512920
once i let the Amanita Muscaria sit in Methanol, and filtered out all the junk. i was wondering about creating a salt from it , if anyone has, and how stable is it? im still a noob in the world of chemisty, this fall im taking Chemisty I. ive been reading a Organic Chemisty book. so im kind of still learning.
(Hive Bee)
06-12-04 11:40
No 512942
Isolating muscimole from A. muscaria is very difficult. I can't imagine any trick that would help.
In the scientific literature it is done with a cromatographyc column.
“The real drug-problem is that we need more and better drugs.” – J. Ott
(Hive Bee)
06-12-04 15:17
No 512962
lets say , i didnt want to isolate the chemical its self , but create a salt of just everything.
(Chief Bee)
06-12-04 20:07
No 513008
(Rated as: good read)
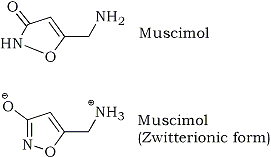
Muscimol does not form salts very well, because while it is usually drawn as being an oxazol-3-one (top picture), this shape forces the molecule to have bonds of varying lenghts and that is very energetically unfavorable. The molecule solves this by moving the carbonyl double bond into the ring to give an aromatic oxazol-3-ol, and because this OH group is rather acidic, the proton will move to the aliphatic nitrogen, forming a zwitterion (a molecule having both positive and negative charges simultaneously).
The Hive - Clandestine Chemists Without Borders
(Hive Bee)
06-12-04 21:49
No 513031
humm , so by creating a salt out of it , the yields would be low. and it wouldnt be an easy proccess for a noob to do? i guess ill just get back to the books.
(Chief Bee)
06-13-04 01:48
No 513049
humm , so by creating a salt out of it , the yields would be low.
...rather that a salt simply won't form.
and it wouldnt be an easy proccess for a noob to do?
You need to make a solvent extract and then purify this by column chromatography, a.a. Flash Chromatography (../rhodium /equipm
The Hive - Clandestine Chemists Without Borders
(Stranger)
06-14-04 13:06
No 513298
Can ibotenic acid form salts?
(Hive Bee)
06-15-04 19:12
No 513585
i was reading this , and i found it quite interesting
http://www.chem.ubc.ca/courseware/121/tu
ive done a paper chromotograph before, but not for seperating, just to see what is in this mysterious mixture.