07-07-04 21:25
No 518090
Hello all
Having some left over NAI from an HI run, I decided to give the MDP-2-P purification via NAI a whirl. It seems pretty straight foward except for one small thing, The write up just says "60gr dirty red ketone", does this mean unwashed ketone by itself not in DCM, or do I take it up into DCM and perform the washes as usual, then do the NAI step. I honestly cannot fathom not washing my goods, but I dont know if the DCM will will interfere with the NAI part. I have searched, but there is not much info on the Hive about NAI, mostly its Bisulfite suff. I did find one post with this same question, but the responses were just ridicule for not using Bisulfite. So the question is still unanswered I really hope someone can help, I mean someone has to have tried this process. Oh, I almost forgot, Im using the Benzo Wacker(in case it helps answer the question better)
Thanks Bees
Rasputin
(Chief Bee)
07-08-04 02:05
No 518141
The write up just says "60gr dirty red ketone", does this mean unwashed ketone by itself not in DCM, or do I take it up into DCM and perform the washes as usual, then do the NAI step. I honestly cannot fathom not washing my goods, but I dont know if the DCM will will interfere with the NAI part.
It means that the ketone is to be fully washed, the DCM dried over a suitable drying agent like MgSO4, and the DCM distilled off (just as in the usual purification procedure, minus the distillation). Compared with the writeup by Hellman (../rhodium /naicom
Please report back your results!
The Hive - Clandestine Chemists Without Borders
(Stranger)
07-14-04 16:06
No 519450
Hi all
As asked, Im posting the results of my expirements with NAI purification of MDP2P. I did the "Time streamlined Benzo Wacker", following the directions exactly, all chemicals were of reagent grade. I did the HCL/H2O flood, and after twenty minutes a layer formed in the bottom. I took it off and added it to solvent, as per the instructions. The first three NaOH washes(3/500 mls) came off jet black. I then proceeded to wash seven more times with NaOH, each time they came off orange. I then washed three more times with DH20(3/500 mls), they also came off orange, but it was a very lighter shade. I then dried with 40 gr MgSO4, for thirty minutes. I then stripped off the solovent. I took 60gr of the "Ketone", added 10gr of NAI, and heated to 100C for ten minutes. None of the NAI dissolved into the "Ketone". I put it into an ice/salt/water bath for ten minutes, still no change. I filtered off the NAI and washed it with a little(50 mls) Xylene( no Toluene available). After it was dried, I added it to three hundered mls of warm DH2O, shook, and nothing sepperated out. All of the NAI dissolved in the DH2O, but there was no "layer of Ketone".
My own conclusions: 1. The process does not work on MDP2P( I dont know why it wouldnt Ketones are Ketones right?)
Or
2. I dont have "Ketone", and its still just Safrole( In the freezer it never froze, it did get very viscous however, isnt that one of the charecteristics of "Ketone"?)
Im not a certified chemist, so I am truly puzzeled, if anyone has any comments or criticism( possitive or negative) Im open and welcome to hear it.
P.S. Rhodium: since your the only one that had any interest in my post, I would really like to hear your input most of all.
Until later: Thank you all, in advance.
Rasputin
(Newbee)
07-15-04 07:31
No 519583
hi, you may try NAI with acetone to see the effect first, then try it again with the ketone.
SWIX is very interested in knowing the result...... SWIX tried using sodium bisulfite for purification and it was quite dirty and obtained balck bisulfaite adduct. so SWIX is planning to try NAI.:) good luck:)
oops i did it again
(Hive Bee)
07-19-04 13:27
No 520320
With the distillation you should have a good idea if you have ketone,as the boiling temp for ketone is much higher than saf.Rhodium has a nomograph on his site to get an idea.
P distills the raw ketone out of the benzo mess,and then re-distills that;when the temperature rises a few degrees, after almost all of the raw ketone is distilled,then it's good enough for P.Is almost clear,and will never freeze.Using this ketone with LaBTop's borohydride reduction always gets P 110g of freebase from 100g ketone.
P tried both of the chemical purifies and just ended up with huge messes and aggravation.
(Stranger)
07-19-04 13:38
No 520321
My assumption is that I dont have Ketone. I ran another Wacker last night, and Im going to work it up tonight. I will post my results, as soon as Im done. I am going to give this process another try(I hate giving up).
P.S. Thanks for replying, I knew I wasnt the only one who was interested in this process.
P.S.2 You said it never freezes, does it get very viscous for you, mine did.
Anxiously awaiting further replies
Rasputin
(Chief Bee)
09-29-04 09:02
No 533758
(Rated as: good read)
The Systems Sodium IodideľAcetone and Sodium IodideľMethyl Ethyl Ketone.
Alan Eric Wadsworth And Harry Medforth Dawson, J. Chem. Soc. 2784-2786 (1926)
In connexion with the purification of acetone for use in experiments on the reaction between acetone and iodine, the authors were led to inquire into the relations underlying the sodium iodide method described by Shipsey and Werner (J. Chem. Soc., 1913, 103, 1255). The inquiry was subsequently extended to ascertain whether the same method could be used for the purification of methyl ethyl ketone. Although the experiments with the latter are not as complete as it was hoped to make them, it seems desirable to place our results on record, more particularly since observations on the system sodium iodideľacetone have just been published by Macy and Thomas (J. Amer. Chem. Soc., 1926, 48, 1547).
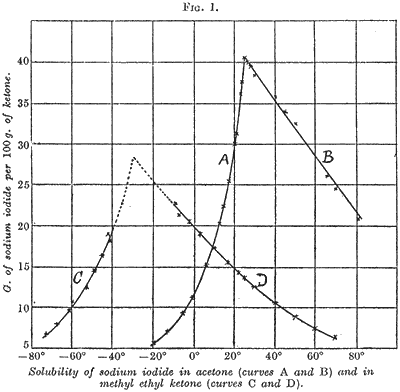
In general, our results for acetone are in agreement with those of Macy and Thomas. At low temperatures the stable solid phase is the compound NaIĚ3Me2C=O, which is converted into the simple iodide at 25.7░C. The solubility of the acetone salt complex increases rapidly with rise of temperature, whereas the simple salt shows a rapid fall.
Corresponding measurements with sodium iodide and methyl ethyl ketone show that this system is very similar to the acetone system. Between -75░C and -45░C, the concentration of the sodium iodide in the liquid phase increases rapidly with rise of temperature, whilst between -10░C and +70░C the proportion of sodium iodide in the saturated solution shows a rapid fall. Over the higher range of temperature, the solid phase is the simple iodide, and the close similarity between the curve systems for the two different ketones suggests that the complex NaIĚ3MeEtC=O is the solid phase at lower temperatures. The point at which this complex is transformed into the simple salt is apparently not far removed from -30░C.
The observations made with methyl ethyl ketone indicate that this also may be purified by the use of sodium iodide. The conditions necessary for such purification are, however, radically different from those prescribed by Lochte (Ind. Eng. Chem., 1924, 16, 956), whose method consists in boiling the moist ketone with excess of sodium iodide, filtering the saturated solution, and allowing it to crystallise at the ordinary temperature. The fact that no result was obtained by Lochte when dry sodium iodide and dry ketone were used is quite in harmony with our observations. The fact that a maximum yield of crystals was obtained by him when the water present was just sufficient to form sodium iodide dihydrate suggests, however, that the crystalline substance which separates out in the procedure recommended by Lochte is hydrated sodium iodide, and it is clear that his method cannot possibly lead to the desired result.
The conditions actually required for the successful and economic purification of methyl ethyl ketone and of acetone can be deduced from the respective solubility curves shown in the diagram, and need not be further discussed. Solubility data taken from the smooth curves are given below for even temperatures.
Solubility Data (g. of sodium iodide per 100 g. of solvent).
(The numbers in red refer to solutions saturated with the sodium iodide-ketone complex.)
Acetone
| Temp. | -20░C | -10░C | 0░C | 10░C | 20░C | 25.7░C | 30░C | 40░C | 50░C | 60░C | 70░C | 80░C |
| Sol. | 5.5 | 7.8 | 11.8 | 18.2 | 30.0 | 40.7 | 39.2 | 35.6 | 32.0 | 28.6 | 25.1 | 21.8 |
Methyl ethyl ketone
| Temp. | -70░C | -60░C | -50░C | -10░C | 0░C | 10░C | 20░C | 30░C | 40░C | 50░C | 60░C | 70░C |
| Sol. | 7.3 | 10.0 | 14.0 | 22.8 | 20.1 | 17.4 | 15.0 | 12.7 | 10.7 | 8.9 | 7.4 | 6.2 |
Experimental
Acetone "A.R." was dried and fractionated; bp 56.1-56.2░C.
Methyl ethyl ketone was dried and purified by repeated fractionation; bp 78-79░C.
Sodium iodide was purified by dissolving it in acetone, crystallising the complex, and removing the acetone by heating to constant weight.
At the lower temperatures, saturated solutions were prepared by mechanical stirring of the solution in contact with the solid phase, a sample being removed for analysis. Precautions were taken to exclude moisture. The data for higher temperatures were obtained by enclosing weighed quantities of sodium iodide and ketone in sealed tubes, the contents of which were effectively shaken while the temperature was very slowly raised or lowered. The temperatures at which the solid phase just disappeared on cooling, or made its appearance on warming, agreed closely, usually within 0.1░C. In other words, there was little tendency towards formation of supersaturated solutions. In the experiments at the highest temperatures, the vapour space was kept as small as possible, and the requisite small correction applied to obtain the amount of solvent actually in the liquid state.
The Hive - Clandestine Chemists Without Borders