09-19-04 08:27
No 532163
http://www.chemikalienlexikon.de/aroinfo
any detailed info about that interesting thing?
(Chief Bee)
09-19-04 12:38
No 532199
Check out this document: ../rhodium /methyle
The Hive - Clandestine Chemists Without Borders
(Stranger)
09-20-04 22:28
No 532447
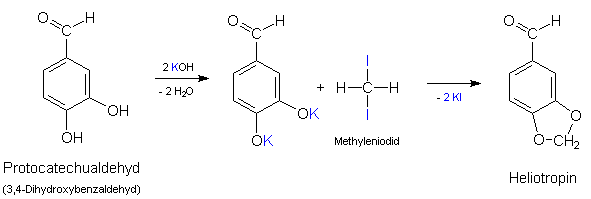
thank you Rhodium; i found it:
When Fittig and Remsen first methylenated a catechol (3,4-dihydroxybenzoic acid), by heating it with methylene iodide and potassium hydroxide, they remarked 'Die Ausbeute war eine sehr geringe'.
../rhodium /methyle
Schade.
(Stranger)
09-21-04 07:28
No 532447
thank you Rhodium; i found it:
When Fittig and Remsen first methylenated a catechol (3,4-dihydroxybenzoic acid), by heating it with methylene iodide and potassium hydroxide, they remarked 'Die Ausbeute war eine sehr geringe'.
../rhodium /methyle
Schade.
(Stranger)
10-12-04 06:36
No 535463
I checked the documents mentioned above, especially:
....A solution of 110 g of catechol, 120 ml of 50% aqueous sodium hydroxide and 200 ml of DMSO was heated to 98°C and stirred at that temperature for 30 minutes. This solution at 98°C. was added over a 30 minute period to a refluxing solution of 120 ml of methylene dichloride in 300 ml of DMSO. Thereafter, the reaction mixture was stirred at reflux for 1.5 hours....
No inert gas is mentioned, not needed?
And how can someone add a solution at 98°C to another, technically, practically?
Any ideas?