10-01-04 19:38
No 534096
hey chem guy, could you relay some info to me regarding Post 52469 (CHEM_GUY: "Re: total P2P synthesis", Novel Discourse).
i have had a look around and can find related articles, but not the procedure you mentioned. (the p2p using lithium, phenyl halide dmso etc)
This seems to be a nice procedure.Thankyou.
edgy
(Chief Bee)
10-02-04 00:56
No 534126
(Rated as: good read)
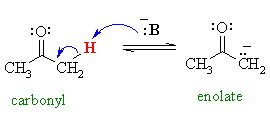
Carey's Online Organic Chemistry, Chapter 18: Enols and Enolates (http://www.chem.ucalgary.ca/courses/351
Enolate phenylacetone synthesis FAQ 1.0 (../rhodium /p2p.en
Post 480727 (Rhodium: "These should interest our acetone enolate bees", Chemistry Discourse)
Post 485886 (Rhodium: "More goodies for the enolate curious", Chemistry Discourse)
Post 392126 (Aurelius: "PhBr and acetone enol reaction to P2P", Methods Discourse)
Post 122752 (dormouse: "what happened to my enolate review?! -drone 342", Serious Chemistry)
Post 108833 (dormouse: "Enolate chemistry is water! P2P from acetone and phenyl halide. -CHEM GUY", Novel Discourse)
Post 315160 (Rhodium: "Somebody! Do it NOW!", Chemistry Discourse)
The Hive - Clandestine Chemists Without Borders
10-03-04 19:54
(Rated as: UTF PM Function)
(Stranger)
10-05-04 10:25
No 534562
I was browsing through Rhodiums web page and found this. It is one of many procedures for p2p . It uses sulfuric acid and 2-Phenyl-propanal (hydratropic aldehyde). I got this idea from jackholes post off of wd; However, no one could seem to substantiate the claim that hydratropic aldehyde is in fact unwatched and used substaintaly in the perfume industry. Well here is the link from Rhodium and part of the post. Not the method you inquired about but possibley easier.
../rhodium /p2p.2pp
9 g of 2-phenylpropanal is slowly added with good stirring during 35 minutes to 40ml concentrated sulfuric acid, while the temperature of the reaction mixture is kept at -16íŽ. After all the 2-phenylpropanal has been added, the mixture is allowed to stand at the same temperature for another 15 minutes, and then the mixture is poured onto crushed ice (100-150g is probably a suitable amount). When the ice has melted, the organics are extracted from the water phase by 3x50ml diethyl ether, the pooled organic phases dried over MgSO4, the ether distilled off and finally the residue is vacuum distilled (bp 91-96íŽ at 11 mmHg) to give 5.6g (62%) of phenyl-2-propanone.
Are you a driver or are you driven will you go to hell or be forgiven