(Member)
04-24-00 16:17
No 108852
(Rated as: good read)
the Hive BB
Novel Discourse
New catalyst system for the Wacker oxidation
profile | register | preferences | faq | search
next newest topic | next oldest topic
Author Topic: New catalyst system for the Wacker oxidation
Cesium
NewBee posted 02-09-2000 10:01 AM
----------------------------------------
Advantages and results: hellman Contact Us | the Hive Powered by: Ultimate Bulletin Board, Version 5.43c
Cesium
NewBee posted 02-09-2000 10:03 AM
----------------------------------------
Dear bees, focus your attention to a recent article, Tetr. Lett., 41 (2000), 99-102,Wacker type oxidation of cyclopentene under dioxygen atmosphere catalyzed by Pd(OAc)2/NPMoV absorbed on activated carbon.
The reaction is carried out under 1 atm of O2 in aqueous acetonitrile or Ethanole acidified by CH3SO3H. Palladium acetate and molybdovanadophosphate supported on activated carbon are used as catalyst-reoxidation catalyst system.
Yelds - 85-90% of cyclopentanone, conversion of cyclopentene over 95% (2h, 50°C, 1 atm O2)
- absence of chlorine ions (compare to conventional PdCl2/CuCl2 system) prevents formation of chlorinated by-products and corrosion of the reactor
- acidification of reaction media (by CH3SO3H) is necessary to achieve conversion and secure reoxidation of heteropolyacid co-catalyst
- different Pd salts were used, acetate showed the best results (over sulphate and chloride)
- absorption of catalyst on activated carbon with high surface area increases yield (probably O2 adsorbed on carbon promotes smooth reoxidation of reduced NPMoV
- catalyst absorbed on activated carbon is easily filtered out of the reaction media and can be reused up to 3 times without lost of activity (in this particular reaction in EtOH)
- reoxidation catalyst NPMoV can be easily prepared (see J. Mol. Catal A Chem. 114 (1996) pp 114-115)
So it seems to me that this new Wacker system is worth of attention of the Hive.
hellman
Hive Bee posted 02-09-2000 08:43 PM
----------------------------------------
Oooooh yeeeeah Baby, LLoooks GRRREAT!!!
Why not just purchase you MDP2P as well,..
Osmium
PimpBee posted 02-10-2000 06:46 AM
----------------------------------------
This is very interesting, because it shows that a Wacker can be performed with only 1 bar O2 in EtOH solvent in a reasonable time (2hrs). The cocatalyst is a bit strange, somebody look up that ref for it, I can't get it.
Reusability is a big advantage. But I don't think that the Pd will be supported on the C too.
The acid, well, it seems like a non-complexing strong acid to me, I bet others can be substituted for it.
Niels Bohr
Hive Bee posted 02-10-2000 06:59 PM
----------------------------------------
For reusability, it looks like nothing will beat the electrolytic oxidations discussed a couple of weeks ago. One method cited reuse of catalyst up to fifty times
All times are CT (US)
next newest topic | next oldest topic
Administrative Options: Close Topic | Archive/Move | Delete Topic
Hop to: Select a Forum or ArchiveList of Forums:General DiscussionNewbie ForumAcquisition DiscourseChemistry DiscourseMethods DiscourseNovel DiscourseCrystal MethSerious Chemistry ForumThe Hive CouchAdmin Chill-out TentList of Archives:Couch ArchivesClassics!Law and OrderThe litter box.misc. PEAs
© Infopop Corporation (formerly Madrona Park, Inc.), 1998 - 2000.
(Chief Bee)
07-15-04 00:31
No 519526
(Rated as: excellent)
Wacker-type oxidation of cyclopentene under dioxygen atmosphere catalyzed by Pd(OAc)2/NPMoV on activated carbon
Arata Kishi, Takashi Higashino, Satoshi Sakaguchi and Yasutaka Ishii
Tetrahedron Letters 41(1), 99-102 (2000) (../rhodium/pdf /wacker.o2-np
Abstract
Wacker-type oxidation of cyclopentene to cyclopentanone under dioxygen atmosphere was successfully achieved by the use of Pd(OAc)2 and molybdovanadophosphate supported on activated carbon, [Pd(OAc)2–NPMoV/C], catalyst. Thus, the reaction of cyclopentene under O2 (1 atm) in aqueous acetonitrile acidified by CH2SO3H in the presence of [Pd(OAc)2–NPMoV/C] at 50°C produced cyclopentanone in 85% yield along with a small amount of cyclopentenone (1%).
The oxidation of terminal alkenes by the Wacker system consisting of PdCl2/CuCl2/O2 to form the corresponding ketones is a well-established method and a very important process in both synthetic and industrial chemistry. However, this catalytic system has disadvantages caused by the chlorine anion which leads to the formation of chlorinated by-products and corrodes the reactor, and it is not suitable for oxidation of higher olefins and cycloalkenes. For this reason much effort has been devoted to the development of aerobic oxidation of cycloalkenes using chloride-free oxidizing systems. Heteropolyacids have frequently been used as the reoxidation catalyst of the reduced Pd(0) to Pd(II) in place of CuCl2.
We would like to report here heterogeneous Wacker-type oxidation of cyclopentene to cyclopentanone by Pd(II) and molybdovanadophosphate (NPMoV) supported on activated carbon (hereafter abbreviated to Pd(II)-NPMoV/C) using molecular oxygen as the terminal oxidant.
In conclusion, Wacker-type oxidation of cyclopentene to cyclopentanone using O2 as terminal oxidant was successfully achieved by using a chloride-free reoxidation system under mild conditions. This method provides an alternative useful direct route for the production of cyclopentenone from cyclopentene easily derived from dicyclopentadiene which is formed in large quantity in the petroleum refining process.
A typical reaction was carried out as follows:
To a suspended solution of [10wt%Pd(OAc)2-15 wt%NPMoV/C] (100 mg) was added cyclopentene (2 mmol) and CH3SO3H (20 mg), and the mixture was stirred under dioxygen atmosphere (1 atm) at 50°C for 6 h (standard conditions). Products were isolated by column chromatography on silica gel with hexane:ethyl acetate (10:1) eluent.
The oxidation of cyclopentene by [10wt%Pd(OAc)2-15wt%NPMoV/C] in acidic CH3CN/H2O under the standard conditions afforded cyclopentanone in 85% yield along with a small amount of cyclopentenone (CPEO) (1%) (run 1). The oxidation was markedly affected by the acidity of the reaction medium. Although p-toluenesulfonic acid had the same effect as CH3SO3H, the reaction in the absence of acid resulted in no formation of cyclopentanone (runs 2 and 3). Similar rate enhancement by the addition of acid is reported by several authors.
Catalyst Preparation:
Molybdovanadophosphate (NPMoV) was prepared according to the literature procedure:
To a solution of NaVO3 (7.32 g, 60 mmol) in water (38 mL) was added Na2MoO4·2H2O (18.22 g, 34 mmol) in water (12 mL). To the resulting solution was added 85% H3PO4 (7.6 g, 66 mmol) in water (10 mL) and the mixture was heated to 95°C under stirring for 1 h. After cooling to 0°C, a saturated aqueous ammonium chloride (150 mL) was added to the solution to give NPMoV which was purified by the recrystallization from water, and dried in vacuo with heating at about 90°C.
The preparation of [Pd(OAc)2-NPMoV/C] was as follows:
Pd(OAc)2 (333 mg) was dissolved in excess acetone and then activated carbon (3 g) was added. After stirring overnight at room temperature, [Pd(OAc)2/C] was obtained in quantitative yield. To a suspended water of the [Pd(OAc)2/C] (3.33 g) was added NPMoV (588 mg), and vigorously stirred for 3 h at room temperature. [Pd(OAc)2-NPMoV/C] was filtered off, washed with water, and dried in vacuo with heating at about 90°C to give [Pd(OAc)2-NPMoV/C] in almost quantitative yield.
____ ___ __ _
Selective Wacker-type oxidation of terminal alkenes and dienes using the Pd(II)/molybdovanadophosphate (NPMoV)/O2 system
Takahiro Yokota, Aki Sakakura, Masayuki Tani, Satoshi Sakaguchi and Yasutaka Ishii
Tetrahedron Letters 43(49), 8887-8891 (2002) (../rhodium/pdf /wacker.o2-np
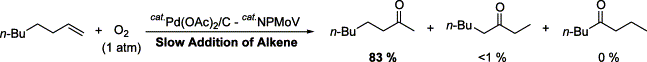
Abstract
Selective Wacker-type oxidation of long-chain terminal alkenes to methylketones was successfully achieved by using Pd(OAc)2/molybdovanadophosphate (NPMoV)/O2 system. The selectivity of the reaction increased by slow addition of the alkenes to the catalytic solution. The oxidation of α,ω-dienes was also examined, and the selectivity of the oxidation was found to depend on the chain length of the dienes used.
____ ___ __ _
This article is referenced from the first two, and details the preparation of molybdovanadophosphate (NPMoV), as well as the making of heterogenous catalysts where activated carbon is coated with either 10% NPMoV or 5% Pd(OAc)2 and also a hybrid catalyst having both 4% Pd(OAc)2 and 6% NPMoV adsorbed to it.
Polyoxometalates in Catalysis
Molybdovanadophosphate (NPMoV)/hydroquinone/O2 system as an efficient reoxidation system in palladium-catalyzed oxidation of alkenes
Takahiro Yokota, Shinya Fujibayashi, Yutaka Nishiyama, Satoshi Sakaguchi and Yasutaka Ishii
Journal of Molecular Catalysis A, 114(1-3), 113-122 (1996) (../rhodium/pdf /wacker.o2-np
Abstract
Molybdovanadophosphate (NPMoV)/hydroquinone/O2 system was found to be an efficient reoxidation system in palladium-catalyzed oxidations of alkenes and related compounds. Thus, acetoxylations of cycloalkenes utilizing molecular oxygen as the final oxidant were cleanly performed using the multicatalytic system consisting of Pd(OAc)2/hydroquinone/NPMoV to form 3-acetoxy-1-cycloalkenes in good yields. For example, cyclopentene and cyclohexene were converted into the corresponding allylic acetates in almost quantitative yields. Omitting hydroquinone from the catalytic system led to low yields of the acetates. Acetoxylation of cyclooctene was satisfactorily achieved by replacing hydroquinone of the multicatalytic system by chlorohydroquinone. Molybdovanadophosphates, which catalyze the smooth dehydrogenation of hydroquinone to benzoquinone with dioxygen, were found to rapidly promote the present Pd(II)-catalyzed acetoxylation of cycloalkenes. By the use of a mixed solvent of ethanol and water under these conditions, Wacker type oxidations of cyclohexene and styrene were accomplished in fair to good yields. Monosubstituted alkenes such as ethyl acrylate and acrylonitrile underwent the acetalization by the present catalytic system to give the corresponding acetals in quantitative yields.
The Hive - Clandestine Chemists Without Borders