12-07-01 04:28
No 245275
crap, at best 15% using 100 g BnZ, 40g l-alanine.
Heat to 140 seems best, extracted into H2o and evaperate, then clean up.
would decarboxylation of a methylamino over the amino maybe improve results?
How about a base and high bp solvent with C5H5N??
as suggested in tfse.
could some one please explain the reaction mechanics for the reaction:
,on heating BzH and DL-alanine directly; PhCH2NH2, PhCH(OH)CHPhNH2 (2 dl-compds.), AcH, and CO2 are formed.
Is this a condensation (no H2o) or a decarboxylation?
what conditions would be pref?
any ed imput would be great, cheers
(Hive Bee)
12-07-01 05:53
No 245287
Give references.
Personnally I don't know of this reaction, and am wondering why you would even attempt it? Get phenylalanine and decarboxylate instead...
(Hive Bee)
12-07-01 10:15
No 245346
complicated rxn mechanism for just words. you'd have to look up the actual article. (btw- your yeild will go up if you calculate according to the converted benzaldehyde, and recover the rest for a second go)
(Stranger)
12-08-01 01:33
No 245572
An otc way to PPA, what I,ve dug up so far
Synthesis of aminoalcohols by aldol condesation of aminoacids with aromatic aldehydes.
The alanine is reduced via -COOH => -CH2-OH
Reaction between aromatic aldehydes and a-amino acids. I. New facts on the Akabori reaction. Takagi, Eiichi. J. Pharm. Soc. Japan (1951), 71 648-51. Journal written in Unavailable.
Abstract
The Akabori reaction (I) (C.A. 41, 3774g) on BzH and dl-MeCH(NHMe)CO2H (II) with and without pyridine and removal of the unreacted BzH by steam distn. gave dl-ephedrine and dl-y-ephedrine. Similarly, direct heating of piperonal and II gave 2 dl-1-(3,4-methylenedioxyphenyl)-2-methyl
Fester5 sites that direct heating of 20g N-methyl-alanine
with 50g Bnz at 150deg untill fizzing stops, produces
12g of a mixture 3g ephedrine and 9g pseudoephedrine isomers.
Reactant BRN 471223 benzaldehyde
1720250 DL-alanine
Product BRN 3196917 (1RS,2RS)-2-amino-1-phenyl-propan-1-ol
-------------------------
Reaction Details
Reaction Classification Preparation
Temperature 140 �C
Other conditions Erwaermen des Reaktionsprodukts mit wss.-aethanol. HCl
Ref. 1 2262852; Journal; Takagi et al.; YKKZAJ; Yakugaku Zasshi; 73; 1953; 1086; Chem.Abstr.; 1954; 12021;
As promised, here are some more refs on the interesting condensation reaction between aromatic aldehydes and glycine/alanine:
BER 25: 3445 (1892) + 52 :1734 ('19)
ANN. 284: 36 + 307: 84
JCS 1943 ('26) + 2600 ('22)
JACS 76: 1322 ('54)
J.PHARM.SOC.JAP. 67: 218 ('47)
Most of the articles are pretty old to say the least but they contain some interesting stuff on the reaction we're interested in here. I'm especially interested in the J.Pharm.Soc.Jap article, which describes the preparation of a methylenedioxy-substituted phenylserine. But the practical way to go is definitely as mentioned in a certain patent, that is using a two-phase solvent system. This prevents the benzylidene phenylserine from crystallising and makes sure that the reaction mixture can be stirred at all times.
After decarboxylation, these phenylserine derivates turn into amino alcohols, the perfect substrates for aminoxazolines. By substituting the benzaldehyde, a lot of phenylserine and amino alcohol derivates can be made and thus a lotta aminoxazolines!
So with some 10x experiments with 20g alanine + 50g BnZ
a massive 15% return sux, any suggestions besides learn how to make nitroethane?
(Hive Bee)
12-04-02 08:24
No 386214
Did I read this correctly then one can then make ephedrine using this method.......well have you tried it and what are the conditions since I can't find the article ,,,anyone?
Akabori
Also known as Akabori-Momotani
Synthesis of aminoalcohols by aldol condesation of aminoacids with aromatic aldehydes.
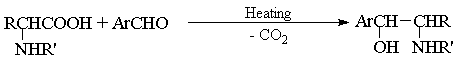
Bibliography
Akabori S., Momotani K., J. Chem. Soc. Japan, 1943, 64, 608; C. A., 1947, 41, 3774
Dose K., Ber., 1957, 90, 1251.
Belikov V. M., Izv. AN SSSR. OHN, 1969, 2536.
This I was able to locate.
(Chief Bee)
12-04-02 08:53
No 386225
Yes, it's correect that you can make ephedrine very simply with the Akabori reaction between Benzaldehyde and N-methylalanine. Ordinary alanine would give phenylpropanolamine.
The only one of the above articles I could locate was J. Am. Chem. Soc. 76, 1322 (1954) (../rhodium/pdf /akabori.phch
I doubt that the original reference has been retrieved as it is written in Japanese, and "IOC" is not likely to answer you either, as he hasn't been logged in since June.