(Hive Addict)
04-14-02 22:25
No 296888
(Rated as: excellent)
Method of preparing Phenylacetaldehyde or Phenylacetic acid
1. Production of Phenylethanol from benzene and ethylene oxide
Patent No US1944959
Abstract
In a suitable container in which there has been placed 500 g of anhydrous AlCl3 there is added 800 g of benzene under stiring, 180 grams ethylene oxide in gaseous form and an equal amount of air are passed through the mixture at 45-55°C until only a small amount of AlCl3 has been left. The reaction container is constantly externally cooled with circulating water to maintain the desired temperature. The temperature is kept preferably withing the range of about substantially 45°C to about subtantially 55°C but as hereafter seen can range from -20°C upwards. The reaction product obtained is treated in the usual way with ice, seperated and vacuum distilled. In this reaction about 173 g phenylethyl alcohol and only 2 g dibenzyl were formed.
2. Ethylene Oxide
Ethanol is mixed with excess of conc sulphuric acid. Ethyl Hydrogen Sulphate is formed in the cold, but on heating to about 170°C it decomposes into ethylene and sulphuric acid.
When ethylene is mixed with air or oxygen and passed over a silver catalyst at 300°C, it is converted into ethylene oxide, a colourless liquid, BP 11C.
3. Oxidisation Of Phenylethanol (phenyl ethyl alcohol)
According to the strength of the oxidising agent used, phenylethanol can be oxidised to phenylacetaldehyde or phenylacetic acid.
Nobodys home
(Hive Bee)
04-14-02 23:13
No 296904
if the temp can range from -22°C on up, then why not chill to 0°C in a salt/water bath? that way everything is liquid phase and the bubbling is no longer a necessity. (good post though)
(Old P2P Cook)
04-14-02 23:50
No 296923
if the temp can range from -22°C on up, then why not chill to 0°C in a salt/water bath? that way everything is liquid phase and the bubbling is no longer a necessity.
Because it probably doesn't work very well at that temperature. The patent states that the best temperature is 45-55°C. When reading patents you have to remember that the purpose of a patent is not to claim just the optimal conditions but, just the opposite, to claim all possible conditions.
(Hive Bee)
04-15-02 06:15
No 297038
Terbium, thanks. that's kinda what aurelius figured but thought it was worth a shot.
(Hive Prodigy)
04-15-02 06:33
No 297042
Would usage of propylene oxide afford P2Pol?
Vivent Longtemps La Ruche!
(Chief Bee)
04-15-02 06:43
No 297044
PP: Yes.
(Hive Prodigy)
04-15-02 06:46
No 297045
But will nonaqueous hypochlorite coordinate with aluminum chloride? (please say no)
Vivent Longtemps La Ruche!
(Hive Bee)
04-15-02 07:25
No 297063
Going for a one-pot-shot at P2P?...
(Hive Prodigy)
04-15-02 07:32
No 297067
Do you remember Post 270904 (PrimoPyro: "Grignard P2Pol In Situ Oxidation", Novel Discourse)? T'would be a neat trick to pull off in one pot, would it not?
PrimoPyro
Vivent Longtemps La Ruche!
(Hive Bee)
04-15-02 07:35
No 297069
PrimoPyro, give it a whirl and post the results. It would be AWESOME to see a synth like that.
(Hive Addict)
04-15-02 15:46
No 297177
The patent also mentions phenylpropyl alcohol and isomers from benzene and propylene oxide.
Propylene Oxide
Propylene could probably be made by the depolymerisation of Polypropylene, or in the usual way with conc sulphuric acid and isopropyl alcohol.
Propylene > Propylene oxide (watch this space)
Phenyl(iso?)propyl alcohol can then be worked up to goodies
Nobodys home
(Hive Addict)
04-15-02 15:56
No 297178
(Rated as: excellent)
When Ethylene glycol is treated with HCl it is converted to Ethylene Chlorhydrin.
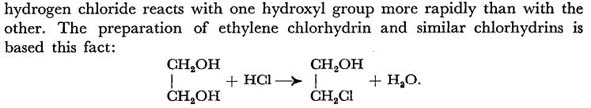
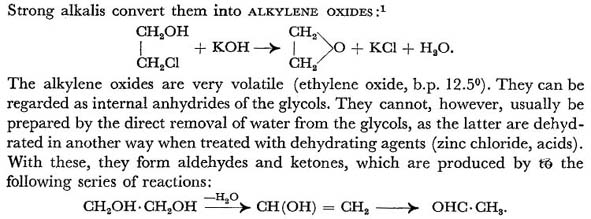
Strong alkali converts Chlorhydrins into Alkylene Oxides
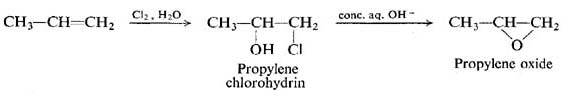
EDIT Chlorine water converts olefins (ethylene/propylene) to chlorohydrins as well.
Nobodys home
(Distinctive Doe)
04-15-02 18:49
No 297226
2,3-propanediol should work with HCl as well. It would bee easier to get than gaseous propylene.
Those who give up essential liberties for temporary safety deserve neither liberty nor safety
(Hive Prodigy)
04-15-02 22:43
No 297341
You people did know that propylene oxide is available as a fuel additive for racing cars by the multigallon drum, did you not?
I mean come on now, what was I thinking when I bought that 5 gallon drum of methanol, 1 gallon of nitromethane, and 1 gallon of propylene oxide for my dragster?
PrimoPyro
Vivent Longtemps La Ruche!
(Old P2P Cook)
04-15-02 22:54
No 297348
I was vaguely under the impression that propylene oxide was not a permitted additive.
(Hive Prodigy)
04-15-02 22:59
No 297351
Well, it is up for sale as an additive in the US, and available for the same at a few Online Places as well.
Check your PMs for details.
PrimoPyro
Vivent Longtemps La Ruche!
(Stoni's sexual toy)
04-15-02 23:19
No 297360
OMG! They sell propylene oxide to the fucking public?? That shit isn't healthy at all.
And I'm not sure if I like to see inexperienced bees working with stuff like ethylene oxide, propylene oxide or even epichlorohydrin either.
I'm not fat just horizontally disproportionate.
(Hive Addict)
05-08-02 04:47
No 306038
Help !
Can any body help me with another name for 2,3-propanediol that foxy mentioned earlier, or even a formula ?
What do you think about the possiblity of changing aluminium chloride for another lewis acid (Iron chloride) in the reaction between benzene and the propylene oxide ?
Don't cry for me I'm the cleaner
(Chief Bee)
05-08-02 06:24
No 306058
Elementary: The proper name is 1,2-propanediol (propylene glycol).
(Hive Addict)
05-09-02 01:02
No 306420
The patent mentions that the usage of propylene oxide will afford phenylpropyl alcohol and isomers.
So I guess the main products will be :
Phenylpropyl Alcohol (Hydrocinnamic Alcohol ?)
Phenylisopropyl Alcohol
Any others ?
So if phenylisopropyl alcohol can be oxidised to 1 Phenyl 2 Propanone (P2P), what does phenylpropyl alcohol get oxidised to ?
And what determines if an secondary alcohol oxidises to an aldehyde(then to an acid) or an ketone ?
Don't cry for me I'm the cleaner
(Hive Prodigy)
05-09-02 01:13
No 306426
Secondary alcohols never become aldehydes or acids. Primary alcohols go through those reactions, and secondary alcohols become ketones.
The other side product of this reaction would be 2-phenyl-1-propanol, which would oxidize to 2-phenylpropanal, which in turn can stil be changed into P2P a la Rhodium's site, with H2SO4 or HgCl2.
The phenylpropyl alcohol would oxidize to phenylpropanal, the aldehyde, or phenylpropionic acid, the carboxylic acid.
PrimoPyro
Vivent Longtemps La Ruche!
(Hive Addict)
05-09-02 01:17
No 306429
The phenylpropyl alcohol would oxidize to phenylpropanal, the aldehyde, or phenylpropionic acid, the carboxylic acid.
So does phenylpropyl alcohol not behave like a secondary alcohol then ?
Don't cry for me I'm the cleaner
(Hive Prodigy)
05-09-02 02:23
No 306465
Phenylpropyl alcohol is a primary alcohol. Phenylpropanol is 1-phenyl-3-propanol, and is a primary alcohol.
Phenylisopropanol is 1-phenyl-2-propanol, and is a secondary alcohol.
2-phenyl-1-propanol is also a primary alcohol, and yields 2-phenylpropanal upon oxidation.
PrimoPyro
Vivent Longtemps La Ruche!
(Old P2P Cook)
05-14-02 08:02
No 308250
Secondary alcohols never become aldehydes or acids.
Not true, especially when the hydrogens next to the alcohol are benzylic, the secondary alcohol can be oxidzed to an acid.
(Hive Prodigy)
05-14-02 11:21
No 308325
But only when accompanied by either a rearrangement or a C-C fission, which I had omitted the possibility of for this thread's topic.
PrimoPyro
Vivent Longtemps La Ruche!
(Moderator)
06-27-04 00:52
No 515621
(Rated as: good read)
This article from Journal of Applied Chemistry USSR (Engl. Trans.) 32, 891-4 (1959) by Etlis and Grobov may bee of interest to some bees

Chemistry is our Covalent Bond