(Stranger)
06-20-02 18:31
No 323392
(Rated as: excellent)
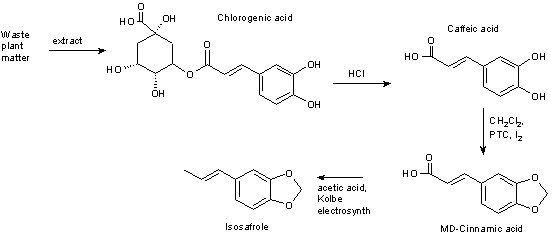
1) Isolate Chlorogenic acid
2) Hydrolysis to caffeic acid (requires HCl)
3) Convert caffeic acid to MD-cinnamic acid (requires DCM, a PTC and a trace of Iodine)
4) reduce the alkene bond
5) dihydro-MD-cinnamic acid + acetic acid gives MDP2P using the Kolbe electrosynth.
Comments please, especially on the Kolbe electrosynth. Has anyone tried this? Could I get away with lead, carbon or stainless steel electrodes? If I must have precious metal electrodes then can I use thinly plated electrodes?
Notes (from Merck XII ed):
1) Isolation of caffeic acid from green coffee,
Wolfrom et al, J. Agr.Food Chem., 8, 58, (1960)
2) Isolation of caffeic acid from roasted coffee,
Krasemann. Arch. Pharm. 293, 721 (1960).
3) Hydroysis of Chlorogenic acid.
a) Fiedler, Arzneimittel-Forsch. 4, 41, (1954)
b) Whiting, Carr. Nature 180, 1479 (1957)
c) Guern. CA 61:9965h (1964)
4) Kolbe Electosynth.
a) B. C. L. Weedon, Quart. Rev. 6, 380 (1952)
b) A. K. Vijh, B. E. Conway, Chem. Rev. 67, 623 (1967)
c) H. J. Schaffer, Comp. Org. Syn. 3, 633-658, (1991)
d) L Eberson in "Organic Electrochemistry" ed Baizer (Dekker, NY 1973)
(Stranger)
06-20-02 18:38
No 323393
Oh s*** excuse my blunder. There is no reduction and the final product will then be isosafrole.
I'll change the gif.
(Old P2P Cook)
06-20-02 20:13
No 323406
It seems that dried plant material is not likely to contain more than 3.5 grams chlorogenic acid per kg.
http://www.startechhealth.com/phenol.htm
Chlorogenic acid is 50% caffeic acid and say that you get a 90% yield on the conversion, so you get about 1.6 grams of caffeic acid per kg of dried plant material. Now you need 3 more chemical steps, say each step gives a 90% molar yield, also the mole weight of safrole is 90% that of caffeic acid so 1.6 gram X (.9)4 = 1.0 gram of isosafrole. So to get 100 grams of isosafrole you are looking at starting with the extraction of 100 kg of dried apples.
I think demethylation of the readily available eugenol via alkali fusion is a much better potential route to isosafrole for those people who can't obtain safrole containing essential oils.
(Stranger)
06-20-02 21:01
No 323418
Agreed - waste plant matter will not do but coffee has much more chlorogenic acid. "13 g roasted coffee per person per day contains about 765 mg of chlorogenic acid" which is nearly 6%, from which you can get about 2.5% caffeic acid.
http://ist-socrates.berkeley.edu/mutagen
(Hive Bee)
06-20-02 23:41
No 323477
Neat idea Bravo!
(Hive Bee)
06-22-02 08:19
No 323933
please to explain the alkali fusion of eugenol. Do you mean demethylation then remethylation of eugenol, or are you talking about connecting the hydroxy group with the methoxy group directly?
Do not go gentle into that good night. Rage, Rage, against the dying of the light. --Dylan Thomas
(Hive Prodigy)
06-22-02 08:35
No 323935
Post 314066 (terbium: "Eugenol demethylation via alkali fusion.", Methods Discourse)
(Distinctive Doe)
06-22-02 09:39
No 323956
That Kolbe step, I have not seen anything to indicate the Kolbe is really a usable/useful reaction. Until I see decent yeilding procedures for asymetric Kolbe reactions published, I'd write it off as a novel "phenomenon", applicable to a PhD reasearch project, not a clandestine synthesis.
Those who give up essential liberties for temporary safety deserve neither liberty nor safety