(Chief Bee)
09-26-02 15:41
No 360747
(Rated as: excellent)
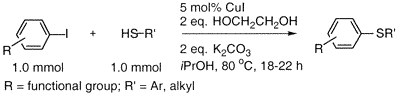
General procedure for copper-catalyzed carbon-sulfur bond formation.
Cu(I) iodide (10 mg, 0.05 mmol), potassium carbonate (276 mg, 2.0 mmol) and the aryl iodide (1.0 mmol) (if a solid) were added to a screw-capped test tube with Teflon-lined septum. The tube was evacuated and backfilled with argon (3 cycles). 2-Propanol (1.0 mL), ethylene glycol (111µL, 2.0 mmol), aryl iodide (1.0 mmol) (if liquid) and the thiol (1.0 mmol) were added by syringes at room temperature. The tube was heated to 80 °C and stirred for 18-24 h. The reaction mixture was then allowed to reach room temperature. Ethyl acetate (approx. 5 mL) and dodecane (227 µL, GC standard) were added. The aliquot was analyzed GC. The reaction mixture was then filtered and concentrated. The crude product was purified by flash column chromatography on silica gel to afford the desired thioether.
Reference: Chemistry Letters 3517-3520 (2002)
../rhodium/pdf /aryliodide.th
../rhodium/pdf /aryliodide.th
(Hive Bee)
09-26-02 15:56
No 360749
Not bad at all.
Catalytic hydrogenation freak
(Stoni's sexual toy)
09-26-02 16:20
No 360758
At least it doesn't seem to require strictly anhydrous conditions as I had expected first.
The Ar only seems to be there to protect the Cu catalyst from oxidation, so it should be possible to conduct that reaction without it on a bigger scale as long as you keep the apparatus closed during most of the reaction, e.g. by a rubber balloon.
(I have the impression most people will do so voluntarily ... sniffle)
I'm not fat just horizontally disproportionate.
(Chief Bee)
09-26-02 16:26
No 360761
What are your experiences with thiol salts? Are they smelly, or just the free thiols?
(Stoni's sexual toy)
09-26-02 16:41
No 360769
While in theory thiol salts should be odorless since they are non-volatile, my very limited experience in thiol chemistry (working in a hood next to someone handling thiols) seems to indicate that the shit always smells somewhat offensive no matter what precausions were taken.
I'm not fat just horizontally disproportionate.
(Hive Addict)
09-26-02 19:22
No 360841
The 2,5-dimethoxythiophenol synth that shulgin describes in pihkal is relatively odorless. trying to make 2,5-dimethoxythiophenol from a grignard on 1-bromo-2,5-dimethoxybenzene and elementary sulfur is an experience in itself. It probably does work, but the end product had such an offensive stench that I didn't dare to put it on the rotovap.
(Chief Bee)
09-27-02 02:47
No 360999
(Rated as: excellent)
2,5-dihydroxyphenylthiosulfate [1]
43.2g benzoquinone (0.4 mol) is dissolved in 150ml glacial acetic acid and heated to 40-50°C. This solution is added to a solution of 150g sodium thiosulfate (1.5 equivalents) in 200ml water at such a rate that the temperature of the aqueous solution does not rise above 10°C. After stirring for a short period, the clear and almost colorless solution is treated with potassium chloride and potassium 2,5-dihydroxyphenylthiosulfate (Ar-S-SO3K) begins to precipitate. After two hours the white precipitate is filtered off, washed with concentrated aqueous KCl, and dried.
The solubility in water is good, but not in alcohol. Reduction with Zn/acid gives the thiophenol as long colorless needles, mp 119-120°C
2,5-dihydroxythiophenol [2]
2.6g Sodium 2,5-dihydroxyphenylthiosulfate (10 mmol) is mixed with 26ml water and 20ml conc HCl. Keeping the temperature of the reaction mixture at 40-50°C, 5g zinc powder is added in small portions with good ventilation, as large amounts of hydrogen and hydrogen sulfide is evolved. When the reaction is over, let the solution cool to room temperature and extract with ether. After evaporating the ether and drying the residue over potassium hydroxide in a dessiccator, the 2,5-dihydroxythiophenol is practically pure. After recrystallization from benzene, the mp is 118°C.
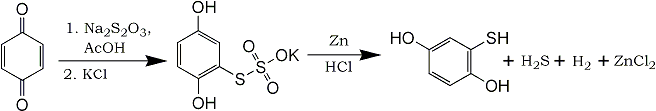
S-monoalkylation of 2,5-dihydroxythiophenol [2]
The article then suggests that dissolving the 2,5-dihydroxythiophenol in methanol (1ml/mmol), adding one equivalent of sodium ethoxide (or NaOH), and then heating this solution together with 1.1 molar equivalent of an alkyl halide in a sealed tube at 80-100°C for 2-4h gives the corresponding
2,5-dihydroxyphenylthioether.
Alternatively 568mg (4 mmol) 2,5-dihydroxythiophenol is dissolved in 10ml 2% aqueous NaOH containing a trace of sodium dithionite (Na2S2O4), and with good stirring and cooling of the reaction mixture, 550mg (0.55ml, 4.4 mmol) dimethylsulfate is added, divided in three portions and with 10 minutes of stirring between each addition. After workup, 2,5-dihydroxythioanisole is isolated in a yield of 300-400mg.
References
[1] Patent DE175070
[2] Helv Chim Acta 30, 578 (1947)
(Hive Addict)
06-01-03 18:36
No 437004
(Rated as: excellent)
After stirring for a short period, the clear and almost colorless solution is treated with potassium chloride and potassium 2,5-dihydroxyphenylthiosulfate (Ar-S-SO3K) begins to precipitate.
Colorless solution? Anyone who has followed this procedure and can second the patent's information that the solution becomes colorless prior to KCl addition? I have tried it three times, and the color always remains a rusty brown/red.
And now some bad news. Reading throug the chemical literature for no obvious reason (certainly not with the intenstion to try ALEPH), I stumbled on the following article: Kinetics of the reaction of p-benzoquinone with sodium thiosulfate. Y Ogata et al. JACS 90(13) (1968) 3469-3472 (../rhodium/pdf /25-dihydroxy
[...]Kinetics - Aqueous solution of p-benzoauinone and of sodium thiosulfate containing acetate buffer in separate flasks were thermostated at a definite temperature. The reaction was started by mixing the two solutions followed by a nimble transfer into a thermostated quartz cell for the UV spectrometry. The unreacted quinone was estimated directly from the optical density at 246 mu, where molecular extinction coefficients of p-benzoquinone, hydroquinone thiosulfate, and thiosulfate are 22000, 2700 and 488 respectively. The concentration of the quinone was determined by the difference method, since absorbances at 246 mu are proportional to their concentrations.
The kinetic reactions were conducted in the presence of excess thiosulfate. The obtained pseudo-first-order rate constant holds good constancy over 90% conversion. Since the rate constants thus obtained were found to be porportional to the initial concentration of sodium thiosulfate, the present reaction was observed to follow second-order-kinetics with respect to both reactants.
Reaction procedure - p-Benzoquinone (2.2 g, 0.02 mol) in 240 mL of water and sodium thiosulfate (5.0 g, 0.02 mol) in 100 mL of water were mixed in the presence of acetate buffer at pH 3.2. The yellow color of the quinone faded out soon after the mixing and then the color of the reaction mixture changed to light brown. The solution was condensed by evaporation under reduced pressure up to ca 15 mL and salting out by addition of KCl (3 g) resulted in the precipitation of potassium hydroquinone thiosulfate. Triplicate recrystallization from water gave ca 0.8 g (15% yield) of fine crystals, mp 225-250°C. Anal. calcd for C6H5O5S2K: C, 27.68; H, 1.94. Found: C, 27.57; H, 2.01.
The IR spectrum of the salt, determined by Nujol paste method, shows peaks at 635, 832, 870, 1035, 1245, and 1660-2000 cm^-1 characteristic of SO, two adjacent ring H, one isolated ring H, SO2, phenolic OH and 1,2,4-trisubstituted ring H, respectively. The UV spectrum in water shows a peak at 313.5 mu (e 4190).
The yield of hydroquinone thiosulfate at pH 2-5, however, is quantitative on the basis of direct spectrophotometry in UV cell instead of isolation of the product. The yield was significantly reduced at pH above 5, e.g. ca 60% at pH 5.6. The main by-product, isolated from the ether extract, was found to be hydroquinone on the basis of IR and UV spectra, but the analysis for all other products was not carried out because of the complexity of products due to the further reaction of hydroquinone and benzoquinone.[...]
I guess the German patent should state that the color upon addition of the benzoquinone/AcOH mixture is colorless, but that the FINAL color is a rusty red/light brown. At least, this article mentions it like this, and it also is what I usually see.
Also, the K salt is too soluble in water to make it precipitating quantitatively. Such a shame... Shulgin reduced the reaction mixture volume and saturates it with a satd. salt solution (according to the drawings, I think he used NaCl). If you do not reduce the volume, you can wave your pretty yields goodbye (in the 30s instead of in the 60s%). I also tried to reduce the volume of the K-salt filtrate, but this gave, off course, alot of KCl as well. I don't think it is worth the extra work to reduce the filtrate's volume as well.
The faster you run, the quicker you die.
(Hive Addict)
06-01-03 19:50
No 437027
Mmmm... I was taking a closer look at Shulgin's ALEPH synthesis (J Pharm Sci 65(10) (1976) 1554), and only now I start wondering why the following step is necessary:
[...] The residue crystallized on standing and was slurried in a saturated salt solution and filtered. The solids were washed with small portions of a saturated salt solution, sucked dry, and finally dissolved in MeOH and filtered through diatomaceous earth. The MeOH filtrate was concentrated in vacuo, yielding 67g of VI as a yellow powder. [...]
Why does he dissolve it in MeOH and isolate the filtrate, instead of using the salt directly in the Zn/HCl reduction? Why does he filter it over celite? One of the previous posts in this thread states the salt's low solubility in alcohol (if I may presume that it will be rather insoluble in MeOH if the same is known for EtOH). Any ideas?
The faster you run, the quicker you die.
(I'm Yust a Typo)
06-01-03 20:01
No 437030
I seem to remember that that concentration step can be pretty difficult. Vapor pressure of water is pretty low compared to standard organic solvents, and you have to evaporate a lot of water. Which means either a rotovap with low vacuum (and the possibility of covering your rotovap with brown/red gunk), or temperatures going to 50-60' (with the possibility of killing your yields).
(Hive Addict)
06-01-03 20:13
No 437034
Which means either a rotovap with low vacuum (and the possibility of covering your rotovap with brown/red gunk), or temperatures going to 50-60' (with the possibility of killing your yields).
I used the rotavap in my latest trial, and no problems with brown/red gunk were encountered. I'm going to do it again right now actually...
The faster you run, the quicker you die.
(Hive Bee)
06-02-03 07:50
No 437138
126:293138
Synthesis of aminomethyl derivatives of 2-hydroxy-5-methoxybenzenethiol.
Movsumzade, M. M.; Novruzova, N. A.; Aliev, Sh. R. (Russia). Zh. Org. Khim., 32(10), 1552-1554 (Russian) 1996 Nauka CODEN: ZORKAE. ISSN: 0514-7492. DOCUMENT TYPE: Journal CA Section: 25 (Benzene, Its Derivatives, and Condensed Benzenoid Compounds)
CGM009979
2-Hydroxy-5-methoxybenzenethiol (I) was prepd. by treatment of 4-methoxyphenol with S2Cl2 and then Zn/HCl. Mannich reactions of I gave aminomethyl derivs. (II; R = NEt2, NBu2, morpholino, piperidino).
Mountain Boy
(Hive Addict)
06-02-03 09:24
No 437149
Seems interesting, although I have no experience with synthesis involving S2Cl2. Is it one of the "better avoid this one from penetrating the nasal cavities"? Also, any HyperLab bee (or bee with some knowledge on the Russian language) interested in digging it up and translate the experimental section?
It might be interesting to develop a so-called shorty ALEPH-2 analogue
On the Shulgin ALEPH synthesis: when salting out with sat'd KCl solution, the formed potassium thiosulfate is filtered (Buechner) and the filtrate provided from extra KCl and gently stirred overnight. There is small amount of precipitate. The color is similar to the first precipitated K salt, but is a fine powder instead of the usual paste. It could be worth it when you have the time, but it certainly is not boosting up your yields.
The faster you run, the quicker you die.
(Chief Bee)
06-02-03 16:22
No 437233
Mountain_Girl: That ref sounds very much like the discussion in Post 207554 (Antoncho: "An easy OTC 2,5-diMeO-phenylmercaptan", Novel Discourse)
(Hive Bee)
09-09-04 17:04
No 530497
(Rated as: good read)
Alkyl- or Arylthiolation of Aryl Iodide via Cleavage of the S-S Bond of Disulfide Compound by Nickel Catalyst and Zinc
Nobukazu Taniguchi
J. Org. Chem.; 2004; ASAP Web Release Date: 27-Aug-2004
DOI:10.1021/jo040184q

Abstract: Various aryl sulfides can be synthesized by nickel-catalyzed alkyl- or arylthiolation of aryl iodide with a disulfide compound. This reaction produces Ni(0) from NiBr2-bpy by the reduction with zinc, and this generated complex works as an activating species to convert ArI into ArSR under neutral conditions. Furthermore, this system enables the use of two RS groups in (RS)2.

Typical Procedure: To a mixture of NiBr2 (6.6 mg, 0.03 mmol), bpy (4.7 mg, 0.03 mmol), zinc (dust) (39.2 mg, 0.6 mmol), and DMF (0.5 mL) were added 2-iodotoluene (65.4 mg, 0.3 mmol) and di-n-butyl disulfide (26.8 mg, 0.15 mmol), and the mixture was stirred at 110 °C for 48 h. After evaporation of the solvent, the residue was dissolved in Et2O, and the resulting mixture was filtered through a Celite pad. The solution was washed with H2O and saturated sodium chloride and dried over anhydrous magnesium sulfate. Chromatography on silica gel (hexane) gave n-butyl 2-tolyl sulfide (45.3 mg, 76%).
The tendency is to push it as far as you can
(Hive Bee)
09-11-04 15:35
No 530842
(Rated as: good read)
A Facile One-Pot Synthesis of Alkyl Aryl Sulfides from Aryl Bromides
Jungyeob Ham, Inho Yang, and Heonjoong Kang
J. Org. Chem., 2004, 69(9), 3236-3239
DOI:10.1021/jo049758h

Abstract: A convenient one-pot synthetic method for the formation of alkyl aryl sulfides from various alkyl halides and lithium aryl thiolates that are prepared in situ by direct halogen-lithium exchange is reported. In particular, the method overcomes many of the problems encountered in previous reports; it is very quick, catalyst-free, and does not involve use of unstable aryl thiols.
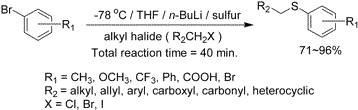
General Procedure for the Formation of Alkyl Aryl Sulfides from Aryl Bromides: To a solution of aryl bromide (2 mmol) in anhydrous THF (15 mL) was added slowly a solution of n-BuLi (1.6 N in hexane, 2 mmol) at -78 °C and the solution was stirred for 15 min under N2 atmosphere. Sulfur powder (2 mmol) was then added. After the reaction mixture changed to a clean yellow solution, alkyl halide (2 mmol) was slowly added and then the reaction mixture warmed to room temperature for 20 min. The reaction was monitored by thin-layer chromatography. After the reaction was completed, it was quenched with aqueous NH4Cl (15 mL). The organic layer was separated and then the aqueous layer (entries 8 and 9 were acidified to approximately pH 2.0 with aqueous 1 N HCl) was extracted with ethyl acetate (2 × 10 mL). The combined extract was washed with water, dried over MgSO4, filtered, and evaporated under reduced pressure to give the crude product. The crude compound was purified by chromatography on silica gel to obtain the desired compound.
The tendency is to push it as far as you can