(Newbee)
09-26-02 21:16
No 360879
(Rated as: good read)
Do any bees know where a synthesis might be found for methylone, 2-methylamino-1-(3,4-methylenedioxypheny
Inhibition of Plasma Membrane Monoamine Transporters by b-Ketoamphetamines,
Cozzi NV, Sievert MK, Shulgin AT, Jacob Peyton III, Ruoho AE
Eur. J. Pharmacol, 1999; 381:63-69 Post 493183 (Rhodium: "Methcathinone & Methylone Pharmacology", General Discourse)
Methcathinone and 2-methylamino-1-(3,4-methylenedioxypheny
Cozzi NV, Sievert MK, Shulgin AT, Jacob Peyton III, Ruoho AE
Soc. Neurosci. Abs, 1998; 24: 381.8
Methcathinone (MCAT) and 2-methylamino-1-(3,4-methylenedioxypheny
Cozzi NV, Shulgin AT, Ruoho AE
Amer. Chem. Soc. Div. Med. Chem. Abs., 1998; 215: 152

(Chief Bee)
09-26-02 21:57
No 360899
3,4-Methylenedioxybenzene + Propionic acid + Polyphosphoric acid -> 3,4-Methylenedioxypropiophenone
3,4-Methylenedioxypropiophenone + N-Bromo-succinimide -> alpha-bromo-3,4-Methylenedioxypropiophenone
alpha-bromo-3,4-Methylenedioxypropiophenone + Methylamine -> Methylone
(Hive Prodigy)
09-26-02 22:20
No 360907
Please tell me you can do the same reaction with formic acid, making piperonal.
Will perform sexual favors for females in exchange for 1,2-dimethylaziridine. PM for details.
(Chief Bee)
09-26-02 22:37
No 360915
Here is a reference for the reaction: ../rhodium /acetoph
I have never heard of the reaction (which essentially is a FC acylation with PPA as the lewis acid) being used with formic acid. There is nothing that would stop any formed piperonal from acylating unreacted benzodioxole, so I guess your suggestion would be a dead end...
The following is a rather old Methylone patent: Patent US3523954
(Stoni's sexual toy)
09-26-02 22:39
No 360916
Formic + strong drying agent -----> CO + CO2
I'm not fat just horizontally disproportionate.
(Official Hive Translator)
09-28-02 08:39
No 361545
1st, as for HCOOH formylations - Primo, believe me, this IS a dead end, i've done once quite a thorough search on this and found nihil - there was one exciting article that Lugh sent me, but it turned out pretty discouraging as well.
2nd, AFAIK, there's no specific need to use NBS for brominating 'propiopiperone'
3rd, this compound has been tested and reported as 'astounding'.
Antoncho
(Old P2P Cook)
09-29-02 06:40
No 361825
Where does the amine go if one adds methylamine to the epoxide formed via peracid oxidation of isosafrole?
(Hive Prodigy)
09-29-02 13:50
No 361922
Since The epoxide opens to give the more stable carbocation, and the amine attaches to the most stable carbocation, I would think the amine would be in the benzyl position, with the alcohol being at the isopropyl position.
PrimoPyro
Will perform sexual favors for females in exchange for 1,2-dimethylaziridine. PM for details.
(Chief Bee)
09-29-02 15:03
No 361935
(Rated as: excellent)
Reactant BRN 171390 5-(1,2-dibromo-propyl)-benzo[1,3]dioxole
Product BRN 165729 1-benzo[1,3]dioxol-5-yl-propan-1-one
No. of Reaction Details 1
Reaction Classification Preparation
Reagent sodium methylate
Other Conditions Erwaermen des Reaktionsprodukts mit Salzsaeure
Note 1 Handbook
Ref. 1 501525; Patent; Hesse; DE 88224; FTFVA6; Fortschr.Teerfarbenfabr.Verw.Industriezw
Ref. 2 500436; Journal; Wallach; Pond; CHBEAM; Chem.Ber.; 28; 1895; 2719.
Ref. 3 500362; Journal; Hoering; CHBEAM; Chem.Ber.; 38; 1905; 3469; CHZEA6; Chem.Zentralbl.; GE; 77; II; 1906; 1223.
Reaction ID 741015
Reactant BRN 6224 benzo[1,3]dioxole-5-carbonitrile
Product BRN 165729 1-benzo[1,3]dioxol-5-yl-propan-1-one
No. of Reaction Details 1
Reaction Classification Preparation
Reagent ethyl magnesium bromide
diethyl ether
Other Conditions Erwaermen des erhaltenen 1-Benzo<1,3>dioxol-5-yl-propan-1-o
Note 1 Handbook
Ref. 1 1997615; Journal; Fuson; Gaertner; Chadwick; JOCEAH; J.Org.Chem.; 13; 1948; 489, 493.
Reaction 15 of 34
Reaction ID 809025
Reactant BRN 82640 5-propenyl-benzo[1,3]dioxole
Product BRN 165729 1-benzo[1,3]dioxol-5-yl-propan-1-one
No. of Reaction Details 1
Reaction Classification Preparation
Reagent phosphorus-pentachloride
Other Conditions Zerlegung des Produkts mit Wasser und Kochen des mit Wasserdampf ueberdestillierten Oels mit alkoholischer Kalilauge
Note 1 Handbook
Ref. 1 500346; Journal; Schimmel & Co.; CHZEA6; Chem.Zentralbl.; GE; 76; I; 1905; 1470.
Ref. 2 501534; Book Review / Secondary Ref.; Schimmel & Co.; Bericht vom April 1905, 49.
Reaction 16 of 34
Reaction ID 1651712
Reactant BRN 115506 benzo[1,3]dioxole
385632 propionyl chloride
Product BRN 165729 1-benzo[1,3]dioxol-5-yl-propan-1-one
No. of Reaction Details 1
Reaction Classification Preparation
Yield 58 percent (BRN=165729)
Reagent SnCl4
Solvent CH2Cl2
Time 1 hour(s)
Temperature 20 C
Ref. 1 5702207; Journal; Daukshas, V. K.; Gaidyalis, P. G.; Pyatrauskas, O. Yu.; Udrenaite, E. B.; Gasperavichene, G. A.; Raguotene, N. V.; PCJOAU; Pharm.Chem.J.(Engl.Transl.); EN; 21; 5; 1987; 341-345; KHFZAN; Khim.Farm.Zh.; RU; 21; 5; 1987; 569-573.
Reaction 17 of 34
Reaction ID 1802483
Reactant BRN 150197 1-benzo[1,3]dioxol-5-yl-propan-1-ol
Product BRN 165729 1-benzo[1,3]dioxol-5-yl-propan-1-one
No. of Reaction Details 1
Reaction Classification Preparation
Yield 64.06 percent (BRN=165729)
Reagent chromium trioxide, concentrated H2SO4
Solvent H2O
acetone
Time 3 hour(s)
Temperature 0 - 20 C
Ref. 1 5677213; Journal; Alesso, Elba N.; Tombari, Dora G.; Iglesias, Graciela Y. Moltrasio; Aguirre, Jose M.; CJCHAG; Can.J.Chem.; EN; 65; 1987; 2568-2574.
Reaction 18 of 34
Reaction ID 1838008
Reactant BRN 165729 1-benzo[1,3]dioxol-5-yl-propan-1-one
1098229 methanol
Product BRN 155294 5-(1-methoxy-propyl)-benzo[1,3]dioxole
No. of Reaction Details 1
Reaction Classification Preparation
Yield 81 percent (BRN=155294)
Reagent NaBH4
Time 30 min
Temperature 20 C
Ref. 1 5519586; Journal; Daukshas, V. K.; Gaidyalis, P. G.; Udrenaite, E. B.; Labanauskas, L. K.; Gasperavichene, G. A.; et al.; PCJOAU; Pharm.Chem.J.(Engl.Transl.); EN; 23; 12; 1989; 990-995; KHFZAN; Khim.Farm.Zh.; RU; 23; 12; 1989; 1466-1470.
Reaction 19 of 34
Reaction ID 1838009
Reactant BRN 165729 1-benzo[1,3]dioxol-5-yl-propan-1-one
505995 formamide
Product BRN 5540275 N-1-(3,4-dimethoxyphenyl)propylformamide
No. of Reaction Details 1
Reaction Classification Preparation
Yield 52.2 percent (BRN=5540275)
Time 8 hour(s)
Temperature 180 - 190 C
Ref. 1 5677213; Journal; Alesso, Elba N.; Tombari, Dora G.; Iglesias, Graciela Y. Moltrasio; Aguirre, Jose M.; CJCHAG; Can.J.Chem.; EN; 65; 1987; 2568-2574.
Reaction 20 of 34
Reaction ID 1838010
Reactant BRN 165729 1-benzo[1,3]dioxol-5-yl-propan-1-one
Product BRN 150197 1-benzo[1,3]dioxol-5-yl-propan-1-ol
No. of Reaction Details 2
Reaction Classification Preparation
Yield 90 percent (BRN=150197)
Reagent LiAlH4
Solvent diethyl ether
Time 30 min
Other Conditions Heating
Ref. 1 5519586; Journal; Daukshas, V. K.; Gaidyalis, P. G.; Udrenaite, E. B.; Labanauskas, L. K.; Gasperavichene, G. A.; et al.; PCJOAU; Pharm.Chem.J.(Engl.Transl.); EN; 23; 12; 1989; 990-995; KHFZAN; Khim.Farm.Zh.; RU; 23; 12; 1989; 1466-1470.
Reaction ID 3824784
Reactant BRN 82642 5-trans-propenyl-benzo[1,3]dioxole
Product BRN 165729 1-benzo[1,3]dioxol-5-yl-propan-1-one
164033 benzo[1,3]dioxol-5-yl-propan-2-one
No. of Reaction Details 3
Reaction Classification Chemical behaviour
Yield 18 percent (BRN=165729)
60 percent (BRN=164033)
Reagent O2
Catalyst PdCl2/CuCl
Solvent dimethylformamide
H2O
Time 10 hour(s)
Temperature 50 C
Other Conditions Reagents
Subject Studied Product distribution
Ref. 1 5618292; Journal; Nagashima, Hideo; Sato, Koji; Tsuji, Jiro; TETRAB; Tetrahedron; EN; 41; 23; 1985; 5645-5652.
Reaction 23 of 34
Reaction ID 5261935
Reactant BRN 165729 1-benzo[1,3]dioxol-5-yl-propan-1-one
Product BRN 8406937 1-benzo[1,3]dioxol-5-yl-2-chloro-propan-
No. of Reaction Details 1
Yield 66 percent (BRN=8406937)
Reaction Classification Preparation
Reagent CuCl2*2H2O
LiCl
Solvent dimethylformamide
Time 6 hour(s)
Temperature 80 C
Reaction Type Chlorination
Ref. 1 6206531; Journal; Zacchino, Susana A.; Lopez, Silvia N.; Pezzenati, German D.; Furlan, Ricardo L.; Santecchia, Carina B.; Munoz, Lorena; Giannini, Fernando A.; Rodriguez, Ana M.; Enriz, Ricardo D.; JNPRDF; J.Nat.Prod.; EN; 62; 10; 1999; 1353 - 1357.
Reaction 24 of 34
Reaction ID 5457190
Reactant $a-<3.4-methylenedioxy-pheny
Product BRN 165729 1-benzo[1,3]dioxol-5-yl-propan-1-one
No. of Reaction Details 1
Reaction Classification Preparation (half reaction)
Reagent aqueous-alcoholic sulfuric acid
Note 1 Handbook
Ref. 1 500305; Journal; Foulds; Robinson; JCSOA9; J.Chem.Soc.; 105; 1914; 1966.
Reaction 26 of 34
Reaction ID 5457192
Reactant 1%1&-ethoxy-isosafrole
Product BRN 165729 1-benzo[1,3]dioxol-5-yl-propan-1-one
No. of Reaction Details 1
Reaction Classification Preparation (half reaction)
Reagent hydrochloric acid
Note 1 Handbook
Ref. 1 500362; Journal; Hoering; CHBEAM; Chem.Ber.; 38; 1905; 3469; CHZEA6; Chem.Zentralbl.; GE; 77; II; 1906; 1223.
Reaction 27 of 34
Reaction ID 5457193
Reactant 1%1&-methoxy-isosafrole
Product BRN 165729 1-benzo[1,3]dioxol-5-yl-propan-1-one
No. of Reaction Details 1
Reaction Classification Preparation (half reaction)
Reagent diluted hydrochloric acid
Note 1 Handbook
Ref. 1 500428; Journal; Tiffeneau; BSCFAS; Bull.Soc.Chim.Fr.; <4> 1; 1907; 1212.
Reactant 1%1&-methoxy-isosafrole
Product BRN 165729 1-benzo[1,3]dioxol-5-yl-propan-1-one
No. of Reaction Details 1
Reaction Classification Preparation (half reaction)
Reagent diluted hydrochloric acid
Note 1 Handbook
Ref. 1 500428; Journal; Tiffeneau; BSCFAS; Bull.Soc.Chim.Fr.; <4> 1; 1907; 1212.
Reaction 28 of 34
Reaction ID 5457194
Reactant BRN 2037554 sulfuric acid
135259 5-prop-1-ynyl-benzo[1,3]dioxole
Product BRN 165729 1-benzo[1,3]dioxol-5-yl-propan-1-one
No. of Reaction Details 1
Reaction Classification Chemical behaviour
Note 1 Handbook
Ref. 1 504625; Book Review / Secondary Ref.; Robinson; Priv.-Mitt..
Reaction 29 of 34
Reaction ID 5457195
Reactant BRN 2036449 2-methyl-2-trideuteriomethoxy-propane
82640 5-propenyl-benzo[1,3]dioxole
Product BRN 165729 1-benzo[1,3]dioxol-5-yl-propan-1-one
No. of Reaction Details 1
Reaction Classification Chemical behaviour
Other Conditions Zerlegung des Reaktionsprodukts mit Wasser und Kochen des mit Wasserdampf destillierten Oels mit alkoh. Kalilauge
Note 1 Handbook
Ref. 1 500429; Book Review / Secondary Ref.; Schimmel & Co.; Bericht vom April 1905, 49; C. 1905 I, 1470.
Reaction 30 of 34
Reaction ID 5457196
Reactant BRN 1098214 hydrochloric acid
9790 1$x-ethoxy-1$x-benzo[1,3]dioxol-5-yl-pro
Product BRN 165729 1-benzo[1,3]dioxol-5-yl-propan-1-one
No. of Reaction Details 1
Reaction Classification Chemical behaviour
Note 1 Handbook
Ref. 1 500362; Journal; Hoering; CHBEAM; Chem.Ber.; 38; 1905; 3469; CHZEA6; Chem.Zentralbl.; GE; 77; II; 1906; 1223.
Reaction 31 of 34
Reaction ID 5457197
Reactant chromic acid
Reactant BRN 150197 1-benzo[1,3]dioxol-5-yl-propan-1-ol
Product BRN 165729 1-benzo[1,3]dioxol-5-yl-propan-1-one
No. of Reaction Details 1
Reaction Classification Chemical behaviour
Note 1 Handbook
Ref. 1 500348; Journal; Mameli; AANLAW; Atti Accad.Naz.Lincei Cl.Sci.Fis.Mat.Nat.Rend.; <5> 13 II; 1904; 320; GCITA9; Gazz.Chim.Ital.; 34 II; 1904; 416.
--------------------------------
Melting Point 1-7 of 7
45 - 46 hexane 1
39 1 2-3
38 2 4
38.5 3 5
38 hexane 4 6
38 - 39 5 7
36 - 37 8
Note 1 Handbook
Note 2 Handbook
Note 3 Handbook
Note 4 Handbook
Note 5 Handbook
Ref. 1 5702207; Journal; Daukshas, V. K.; Gaidyalis, P. G.; Pyatrauskas, O. Yu.; Udrenaite, E. B.; Gasperavichene, G. A.; Raguotene, N. V.; PCJOAU; Pharm.Chem.J.(Engl.Transl.); EN; 21; 5; 1987; 341-345; KHFZAN; Khim.Farm.Zh.; RU; 21; 5; 1987; 569-573.
Ref. 2 501525; Patent; Hesse; DE 88224; FTFVA6; Fortschr.Teerfarbenfabr.Verw.Industriezw
Ref. 3 500436; Journal; Wallach; Pond; CHBEAM; Chem.Ber.; 28; 1895; 2719.
Ref. 4 735496; Journal; Angeli; GCITA9; Gazz.Chim.Ital.; 22 II; 1892; 479; CHBEAM; Chem.Ber.; 25; 1892; 1960.
Ref. 5 504175; Journal; Ciamician; Silber; GCITA9; Gazz.Chim.Ital.; 23 II; 1893; 195, 202; GCITA9; Gazz.Chim.Ital.; 24 I; 1894; 431, 541.
Ref. 6 514935; Journal; Hillmer; Schorning; ZPCLAH; Z.Phys.Chem.(Leipzig); <A> 168; 1934; 81, 104.
Ref. 7 1997615; Journal; Fuson; Gaertner; Chadwick; JOCEAH; J.Org.Chem.; 13; 1948; 489, 493.
Ref. 8 5677213; Journal; Alesso, Elba N.; Tombari, Dora G.; Iglesias, Graciela Y. Moltrasio; Aguirre, Jose M.; CJCHAG; Can.J.Chem.; EN; 65; 1987; 2568-2574.
-----------------------
Boiling Point 1-2 of 2
153 - 154 13 1 1-2
128 3.5 2 3
Note 1 Handbook
Note 2 Handbook
Ref. 1 501525; Patent; Hesse; DE 88224; FTFVA6; Fortschr.Teerfarbenfabr.Verw.Industriezw
Ref. 2 500436; Journal; Wallach; Pond; CHBEAM; Chem.Ber.; 28; 1895; 2719.
Ref. 3 519826; Patent; Winthrop Chem. Co.; US 1964973; 1932.
--------------
CAS Registry Number 41103-34-8
Chemical Name 5-(1,2-dibromo-propyl)-benzo[1,3]di
Isosafroldibromid
Autoname 5-(1,2-dibromo-propyl)-benzo[1,
Molecular Formula C10H10Br2O2
Molecular Weight 322.00
Melting Point 1-4 of 4
51 1 1
52 - 53 2 2-3
52 - 53 3 2
51 - 52 pentane 4 4
Note 1 Handbook
Note 2 Handbook
Note 3 Handbook
Note 4 Handbook
Ref. 1 500366; Journal; Hoering; Baum; CHBEAM; Chem.Ber.; 42; 1909; 3080.
Ref. 2 500364; Journal; Waterman; Priester; RTCPA3; Recl.Trav.Chim.Pays-Bas; 48; 1929; 941.
Ref. 3 1534557; Journal; Mannich; ARPMAS; Arch.Pharm.(Weinheim Ger.); 248; 1910; 143; CHZEA6; Chem.Zentralbl.; GE; 80; I; 1909; 923.
Ref. 4 512137; Journal; Naves; Ardizio; BSCFAS; Bull.Soc.Chim.Fr.; 1957; 1053, 1056.
Reaction 5 of 20
Reaction ID 186589
Reactant BRN 171390 5-(1,2-dibromo-propyl)-benzo[1,3]dioxole
Product BRN 135259 5-prop-1-ynyl-benzo[1,3]dioxole
No. of Reaction Details 1
Reaction Classification Preparation
Reagent diethylene glycol
potassium hydroxide
Note 1 Handbook
Ref. 1 512718; Journal; Nelb; Tarbell; JACSAT; J.Amer.Chem.Soc.; 71; 1949; 2936.
Reaction 6 of 20
Reaction ID 186590
Reactant BRN 171390 5-(1,2-dibromo-propyl)-benzo[1,3]dioxole
Product BRN 150217 5-(2-bromo-propenyl)-benzo[1,3]dioxole
No. of Reaction Details 1
Reaction Classification Preparation
Other Conditions Bei der Destillation unter vermindertem Druck
Note 1 Handbook
Ref. 1 500305; Journal; Foulds; Robinson; JCSOA9; J.Chem.Soc.; 105; 1914; 1966.
Reaction 7 of 20
Reaction ID 186591
Reactant BRN 171390 5-(1,2-dibromo-propyl)-benzo[1,3]dioxole
Product BRN 165729 1-benzo[1,3]dioxol-5-yl-propan-1-one
No. of Reaction Details 1
Reaction Classification Preparation
Reagent sodium methylate
Other Conditions Erwaermen des Reaktionsprodukts mit Salzsaeure
Note 1 Handbook
Ref. 1 501525; Patent; Hesse; DE 88224; FTFVA6; Fortschr.Teerfarbenfabr.Verw.Industriezw
Ref. 2 500436; Journal; Wallach; Pond; CHBEAM; Chem.Ber.; 28; 1895; 2719.
Ref. 3 500362; Journal; Hoering; CHBEAM; Chem.Ber.; 38; 1905; 3469; CHZEA6; Chem.Zentralbl.; GE; 77; II; 1906; 1223.
Reaction 8 of 20
Reaction ID 186592
Reactant BRN 171390 5-(1,2-dibromo-propyl)-benzo[1,3]dioxole
Product BRN 171391 1-benzo[1,3]dioxol-5-yl-2-bromo-propan-1
No. of Reaction Details 2
Reaction Classification Preparation
Reagent aqueous acetone
marble
Note 1 Handbook
Ref. 1 500356; Patent; Hoering; DE 174496.
Ref. 2 500362; Journal; Hoering; CHBEAM; Chem.Ber.; 38; 1905; 3469; CHZEA6; Chem.Zentralbl.; GE; 77; II; 1906; 1223.
Reaction 9 of 20
Reaction ID 809028
Reactant BRN 82640 5-propenyl-benzo[1,3]dioxole
Product BRN 171390 5-(1,2-dibromo-propyl)-benzo[1,3]dioxole
No. of Reaction Details 3
Reaction Classification Preparation
Reagent diethyl ether
bromine
Note 1 Handbook
Ref. 1 500360; Journal; Wallach; Pond; CHBEAM; Chem.Ber.; 38; 1905; 2719.
Reaction 14 of 20
Reaction ID 5400404
Reactant BRN 171390 5-(1,2-dibromo-propyl)-benzo[1,3]dioxole
Product 1%2&-bromo-isosafrole
No. of Reaction Details 1
Reaction Classification Chemical behaviour (half reaction)
Other Conditions Bei der Destillation unter vermindertem Druck
Note 1 Handbook
Ref. 1 500305; Journal; Foulds; Robinson; JCSOA9; J.Chem.Soc.; 105; 1914; 1966.
Reaction 15 of 20
Reaction ID 5461129
Reactant BRN 3587193 Br2
1696894 diethyl ether
82640 5-propenyl-benzo[1,3]dioxole
Product BRN 171390 5-(1,2-dibromo-propyl)-benzo[1,3]dioxole
No. of Reaction Details 1
Reaction Classification Chemical behaviour
Note 1 Handbook
Ref. 1 500436; Journal; Wallach; Pond; CHBEAM; Chem.Ber.; 28; 1895; 2719.
Reaction 16 of 20
Reaction ID 5461130
Reactant cis-isosafrole
Product BRN 171390 5-(1,2-dibromo-propyl)-benzo[1,3]dioxole
No. of Reaction Details 1
Reaction Classification Preparation (half reaction)
Reagent diethyl ether
bromine
Note 1 Handbook
Ref. 1 512137; Journal; Naves; Ardizio; BSCFAS; Bull.Soc.Chim.Fr.; 1957; 1053, 1056.
Reaction 17 of 20
Reaction ID 5461132
Reactant trans-isosafrole
Product BRN 171390 5-(1,2-dibromo-propyl)-benzo[1,3]dioxole
No. of Reaction Details 1
Reaction Classification Preparation (half reaction)
Reagent diethyl ether
bromine
Temperature -10 C
Note 1 Handbook
Ref. 1 512137; Journal; Naves; Ardizio; BSCFAS; Bull.Soc.Chim.Fr.; 1957; 1053, 1056.
Reaction 18 of 20
Reaction ID 5574136
Reactant BRN 171390 5-(1,2-dibromo-propyl)-benzo[1,3]dioxole
Reactant isosafrole bromohydrin
Product BRN 357396 bis-(1-benzo[1,3]dioxol-5-yl-2-bromo-pro
No. of Reaction Details 1
Reaction Classification Preparation
Other Conditions im verschlossenen Gefaess
Note 1 Handbook
Ref. 1 969731; Journal; Mannich; Schmitt; ARPMAS; Arch.Pharm.(Weinheim Ger.); 1928; 80; CHZEA6; Chem.Zentralbl.; GE; 98; I; 1927; 951.
Reaction 19 of 20
Reaction ID 5574947
Reactant BRN 171390 5-(1,2-dibromo-propyl)-benzo[1,3]dioxole
Product 1%2&-bromo-isosafrole
Product BRN 3587158 hydrogen bromide
No. of Reaction Details 1
Reaction Classification Chemical behaviour
Note 1 Handbook
Ref. 1 500364; Journal; Waterman; Priester; RTCPA3; Recl.Trav.Chim.Pays-Bas; 48; 1929; 941.
(Hive Bee)
10-01-02 11:30
No 362671
(Rated as: excellent)
Rapid microwave-promoted synthesis of functionalised benzophenones
David A. Learmonth
Synthetic Communications, Volume 32, Issue 18, Pages 2757-2762
Abstract
Several functionalised benzophenone derivatives have been conveniently obtained in moderate to good yield via the rapid, microwave-promoted Friedel–Crafts acylation of activated arenes with benzoic acids in polyphosphoric acid medium.
...
Reactions were run in open borosilicate round-bottom glass vessels at atmospheric pressure in a microwave oven adapted for organic synthesis (focussed microwaves on reaction vessel; continuous un-pulsated power regulation; microwave cavity dimensions: height 210 mm, width 290 mm, depth 320 mm; incorporated magnetic stirrer, infra-red temperature sensor and overfield sensor).
A typical experimental procedure for the microwave-promoted synthesis of benzophenones is exemplified by the following example:
3,4-Dimethoxy-4-methyl-benzophenone (Run 1): Polyphosphoric acid (15 g) was added to veratrole (1.0 g, 7.25 mmo1) and p-toluic acid (1.08 g, 7.97 mmo1) in an open 50 mL round bottom flask equipped with a magnetic stirring bar. The mixture was placed in the cavity of the microwave oven and irradiated at 100% power for 45 s, with the maximum allowed temperature set at 120 deg C. The resulting deep red mixture was allowed to cool, and then poured onto ice/water (100 mL). The precipitate was filtered off, washed with water and dried in air. Recrystallisation (iPrOH) afforded the product as beige crystals, 1.52 g, (82%) of m.p. 127-128 deg C.
(Chief Bee)
10-01-02 14:34
No 362695
(Rated as: excellent)
Seems like I forgot to post this forensic article about methylone analogs:
The characterization of some 3,4-methylenedioxycathinone (MDCATH) homologs
Terry A. Dal Cason
Forensic Science International Volume 87, Issue 1, 9-53 (1997) (../rhodium/pdf /methylenedio
DOI:10.1016/S0379-0738(97)02133-6
Abstract
In the past 35 years, a wide variety of illicit drugs have appeared in the clandestine market. Many of these compounds are based on the structure of amphetamine (1-phenyl-2-aminopropane) to which various functional or structural groups have been added. Previous modifications to the amphetamine molecule include addition of a methylenedioxy bridge to give 3,4-methylenedioxyamphetamine, and attachment of alpha,beta-keto oxygen to yield cathinone. A chemical synthesis integrating the salient functional/structural groups of these two classes of amphetamine analogs results in manufacture of methylenedioxycathinone (MDCATH). In each instance, N-alkylation of these analogs provides a series of homologs. Furthermore, many of these analogs/homologs meet several criteria which typically support the clandestine laboratory synthesis of novel illicit drugs (`designer drugs'). The MDCATH analogs represent a potentially new series of `designer drugs' whose chemical characteristics have not previously been reported. Appropriate selection of analytical, chemical and physical tests will enable rapid identification of these analogs by a comparative analysis using the data provided.
(Chief Bee)
12-09-03 21:17
No 475682
(Rated as: excellent)
Cathinone: An investigation of several N-alkyl and methylenedioxy-substituted analogs
DalCason TA, Young R, Glennon RA
Pharmacology Biochemistry and Behavior 58(4), 1109-1116 (1997) (../rhodium/pdf /cathinone-me
DOI:10.1016/S0091-3057(97)00323-7
Abstract
Structurally, methcathinone is to cathinone what methamphetamine is to amphetamine. Due to increased interest in the abuse of suck agents we wished to determine if certain derivatives of cathinone would behave in a manner consistent with what is known about their amphetamine counterparts; that is, can amphetamine structure-activity relationships be extrapolated to cathinone analogs? As expected on the basis of known structure-activity relationships for amphetaminergic agents, both N-monoethylcathinone and N-mono-n-propylcathinone (N-Et CAT and N-Pr CAT; ED50 = 0.77 and 2.03 mg/kg, respectively) produced amphetamine-like stimulus effects in rats trained to discriminate 1 mg/kg of (+)amphetamine from Vehicle and were somewhat less potent than racemic methcathinone. In contrast, (-)N,N-dimethylcathinone or (-)Di Me CAT (ED50 = 0.44 mg/kg) was more potent than expected; although (+)N,N-dimethylamphetamine is sevenfold less potent than (+)methamphetamine, (-)Di Me CAT is only about 1.6-fold less potent than (-)methcathinone, and is essentially equipotent with (-)cathinone. In addition, although it has been previously demonstrated that 1-(3,4-methylenedioxyphenyl) -2-aminopropane (MD A) results in stimulus generalization in rats trained to discriminate (+)amphetamine or DOM from vehicle; the cathinone counterpart of MDA (i.e., MDC) resulted in partial (maximum: 58%) generalization in (+)amphetamine-trained animals, and failed to produce >7% DOM-appropriate responding in rats trained to discriminate DOM from vehicle. On the other hand, the N-methyl analog of MDC (i.e., MDMC) behaved in a manner similar to that of the N-methyl analog of MDA (i.e., MDMA); that is, st (+)amphetamine stimulus (MDMC: ED50 = 2.36 mg/kg) but not a DOM stimulus generalized to MDMC. In MDMA-trained rats, stimulus generalization occurred both to MDC and MDMC (ED50 = 1.64 and 1.60 mg/kg, respectively). Although this and previous studies have demonstrated that significant parallelisms exist between the structure-activity relationships of amphetamine analogs and cathinone analogs, we now report several unexpected qualitative and/or quantitative differences. It is suggested that caution be used in attempting to draw conclusions or make predictions about the activity and potency of novel cathinone analogs by analogy to the structure-activity relationships derived from amphetamine-related agents; it would appear that each new cathinone analog will require individual investigation.
Abstract posted earlier in Post 108548 (dormouse: "Methylenedioxy-cathinone analogs -Lone Deranger", Novel Discourse)
(Chief Bee)
12-09-03 21:48
No 475692
(Rated as: excellent)
A two-step method for the preparation of homochiral cathinones
M. Osorio-Olivaresa, M. C. Rezende, S. Sepúlveda-Bozab, B. K. Cassels, R. F. Baggio, J. C. Muñoz-Acevedoe
Tetrahedron: Asymmetry 14(11), 1473-1477 (2003) (../rhodium/pdf /two-step.chi
DOI:10.1016/S0957-4166(03)00317-3
Abstract
A simple method for the preparation of homochiral ring-substituted 1-aryl-2-aminopropanones 2 (`cathinones´) is described, involving initial Friedel–Crafts acylation of aromatics with (S)- or (R)-N-trifluoroacetylalanyl chloride, followed by acid hydrolysis of the intermediate trifluoroacetamido intermediates 1, for which X-ray diffraction analysis confirmed the structures.
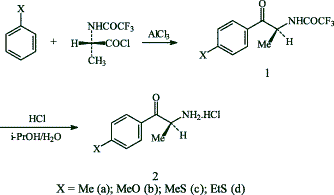
(S)-N-Trifluoroacetylalanine
1,1,3,3-Tetramethylguanidine (3.75 mL, 30 mmol) was added to a suspension of L-alanine (2.0 g, 22 mmol) in MeOH (11 mL). After 5 min, ethyl trifluoroacetate (3.3 mL, 28 mmol) was added and the reaction was stirred for 4 h at room temperature. The solvent was then removed by rotary evaporation and the residue dissolved in H2O (35 ml) and acidified with concentrated HCl (4 mL). After stirring for 15 min, the mixture was extracted with EtOAc (2×30 mL) and the organic layers were combined and washed with brine (30 mL), dried over Na2SO4 and rotary evaporated to give a solid which was washed with n-hexane and dried to afford the crude amide, mp 62–64°C, yield 3.5 g (86%), sufficiently pure for all subsequent uses.
General procedure for the preparation of (S)-2-trifluoroacetamido-1-aryl-1-propan
To a stirred suspension of (S)-N-trifluoroacetylalanine (1 g, 5.4 mmol) in dry CH2Cl2 (20 mL), cooled to 0°C in an ice-water bath, was added oxalyl chloride (1.1 mL, 12.8 mmol) followed by pyridine (1 drop). The reaction mixture was allowed to warm gradually to room temperature and was then stirred further for 5 h. The solvent and excess oxalyl chloride were removed by rotary evaporation at 35°C to afford the crude acid chloride. To this chloride was then added with stirring a solution of the aromatic compound (5.4 mmol) in CH2Cl2 (5 mL), followed by AlCl3 (0.72 g, 5.4 mmol) and the resulting mixture was allowed to react for 18 h. The reaction mixture was then cooled in an ice-water bath and slowly quenched with 1N HCl (30 mL) and CH2Cl2 (30 mL). The aqueous layer was extracted with CH2Cl2 (2×30 mL) and the organic layers were combined, dried over Na2SO4, and rotary evaporated to give the crude product 1, which was crystallized in hexane.
General procedure for the preparation of (S)-2-amino-1-aryl-1-propanone hydrochlorides 2
The (S)-2-trifluoroacetamido-1-aryl-1-propan
(Chief Bee)
10-22-04 22:02
No 537261
(Rated as: excellent)
α-Amino Acids as Chiral Educts for Asymmetric Products. Amino Acylation with N-Acylamino Acids
Thomas F. Buckley III and Henry Rapoport
J. Am. Chem. Soc., 103, 6157-6163 (1981) (../rhodium/pdf /fc-acylation
Abstract
α-N-Acylamino acids have been developed as useful reagents for the preparation of optically pure α-aminoalkyl aryl ketones. Protection of the amino group as either the ethoxycarbonyl or benzenesulfonyl derivative allows alanine to serve as an effective educt for the chirally specific synthesis of a variety of structures containing the phenylethylamine backbone. Benzene undergoes Friedel-Crafts acylation with the N-acylalanine acid chloride? Catalyst complexation with oxygenated aromatics, however, prohibits acylation of aryl ethers. An arylmetallo reaction scheme overcomes this problem and also affords regiospecificity not attainable in conventional acylations. As examples, optically pure ephedrines and amphetamines were directly synthesized without recourse to resolution since the chirality of the amino acid educt was entirely conserved throughout the process.
The Hive - Clandestine Chemists Without Borders
(Stranger)
10-28-04 19:33
No 538421
I have tried methylone (MDMCAT) for myself; in fact, I did a total of 6 grams of it, and I have heard of someone at the hive mentioning having intoxicating himself on MDCAT, but does anyone here have any information or personal experience with the N-ethyl homologue of methylone, MDECAT?
(Hive Bee)
10-29-04 09:28
No 538568
rhodium; so I take it from the article that the following could be a viable route?
DL-alanine + benzenesulfonyl chloride -> N-benzenesulfonylalanine
N-benzenesulfonylalanine -> N-benzenesulfonylalanyl chloride
benzodioxole -> 3,4-methylenedioxyphenylmagnesium bromide
3,4-methylenedioxyphenylmagnesium bromide (2 molar eq.) + N-benzenesulfonylalanyl chloride -> 2-((Benzenesulfonyl)amino)-1-(3,4-methyl
2-((Benzenesulfonyl)amino)-1-(3,4-methyl
2-((Benzenesulfonyl)-methylamino)-1-(3,4
- phenethylman -