11-19-02 16:54
No 381200
I was at 1st hesitant as to where to place this post. Finally, being unable to choose between three different threads, I decided to post it separately
This post is about making and using substituted alkali metal amides. As we all know, the range of application of 'regular' sodium amide is very broad - i'll get to it further on.
Unfortunately, most of the procedures involving it make use of liquid ammonia as both the reagent and solvent (and hence, need for conditions poorly suitable for a kitchen chemist).
However, if one was to use a soln of Na amide in some higher-boiling amine (say, diethylamine , which is dirt cheap and unconspicious), one would arrive at a very similar conditions - except all of it could bee made at RT!
Here are a few examples which these conditions can bee used for:
1) Originally the info you're about to read was fetched from the depths of EspaceNet and USPat.gov as a tribute to the outstanding (IMHO) fallen_Angel's idea, which for some reason was so undeservedly misappreciated at the 'big' Hive : Post 379214 (fallen_Angel: "P2P - new idea.", Novel Discourse)
You can see that Na monoacetylide used in the 1st step of the synth proposed by Angel is formed by rxn of acetylene w/NaNH2, which in turn is formed in situ by dissolving Na in liquid ammonia (aka 'anny' as i recently learned from Lili's most excellent Abbreviations' dictionary)
The same reaction can bee achieved using some other alkali metal amides (the latter, say, Na diethylamide or some such - being even weaker acid than NaNH2, they will react yet more readily w/acetylene. However, this topic will later bee elaborated in the proper thread.
2) The second immediate thought that comes to mind is the most elegant route to Ph-acetonitriles found by Chimimanie, Post 374179 (Chimimanie: "Important advance in the propagation of 2C-H", Novel Discourse).
It isn't completely clear as to how the 'harsher' conditions (stronger base, higher temp) will affect the rxn's selectivity, but certainly, it looks worthwhile, doesn't it?
3) And last, but note least - the oh-so-much-talked-over-and-over-again 'enolate route', which also makes use of strong bases - and again, the stronger the base, the better. If you look thru all the xamples posted by Drone several years ago, you'll see that in the very last example they use a strong base and liquid ammonia as the solvent. Looks similar, doesn't it?
But again, here i have an altogether separate rabbit up my sleeve whom i'll pull out later.
Ok. Now - what it's all about. Substituted sodium amides can't bee made by direct rxn of the metal with the amine (and i'm unclear as to if simply dissolving Na in anny
However, there is a trick. As it turns, alkali metals can form addition compounds with certain things - unsaturated hydrocarbons and alkyl/aryl halides. These then react w/any amine which happens to bee at hand to give the desired amide.
Note that here we aren't talking of making butyl or phenyl lithium - all of the said is made in one pot, the addition complex being consumed as soon as it forms.
Here go (yes, finally!
1. Patent US2141058, Example 1.
128g of naphtalene, 14g of small pieces of lithium and 150g of diethylamine are filled up in a nitrogen atmosphere with ether to 1 liter. After a few minutes a vigorous rxn takes place and the ether starts to boil. The rxn is brought to an end w/gentle stirring. The metal dissolves completely forming a colorless, almost clear solution.
2. Patent US2141058, Example 3.
To 112 parts chlorobenzene or 92 parts butyl chlorode in 500 parts benzene are added under nitrogen atm. 100 parts of dry cyclohexylamine. 46 g of Na metal wire are pressed into this mixture, and upon vigorous stirring the metal is slowly transformed into Na cyclohexylamide and NaCl, the latter being suspended in benzene (note that in this case, half of the metal is waisted as NaCl).
3. Patent US6169203, Example 7.
A reactor was charged with 1.0 mole of lithium metal and 1.0 mole of di-isopropylamine were charged to a reactor. Over a 2.5 hour period, 0.5 moles of isoprene were added. The reaction temperature ranged from approximately 21 to 45 DEG C. over the addition of isoprene. The reaction proceeded best at temperature over 40 DEG C. The reaction mixture became thick as the reaction proceeded. The reaction proceeded to greater than 90% completion
Here you go!
What do you think – what kind of moisture sensitivity can one expect from such a rxn? Will it bee as picky as Grignar, or not so much since any traces of water will bee destroyed by the alkali metal?
Dear maitres, please express your opinions!
In any case, bees, just let me know what you think
Antoncho
(Stoni's sexual toy)
11-19-02 19:36
No 381264
I once had to prepare some sodium naphtalide in THF, and also solid cyclopentadienyl-sodium. The former is deeply green coloured, so once the flask turns green you know it worked and the reaction is finally anhydrous enough to proceed on its own. It is not easy to start it, even when using argon, rigorously dried solvents and professional schlenk glassware. Ultrasound helps a lot to get this one started, but I doubt that anyone is able to do this in a ghetto lab with jars etc. I'd say it's definitely harder to do than most Grignard reactions, but my experience is limited.
Cyclopentadienyl-sodium is a colourless/white solid if I remember correctly, and it was much easier to prepare and handle.
I'm not fat just horizontally disproportionate.
(Stranger)
11-20-02 01:10
No 381371
Hmm A synthesis of LDA! I was just searching this four hours ago, thank Antoncho! Thats a nice present!
Anyway for the lithium diethylamide, I will try to read more in details all the new stuff I took and try to fetch some article with aryne from substituted alkali amides and then when I will have a more global vision of all that stuff I will post what I think about it. But it may work
(Moderator)
11-20-02 01:55
No 381402
One consideration should bee the fact that sodium amide reacts with glass, thus the traditional method was performed in iron
(Moderator)
11-22-02 01:36
No 381995
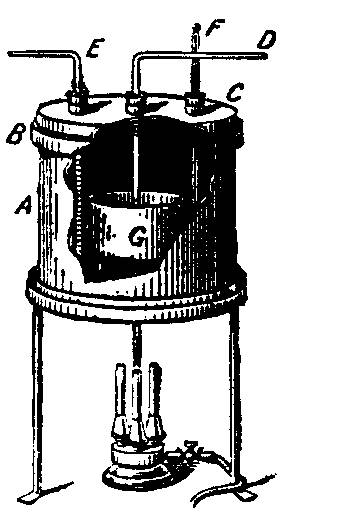
From JACS 26 577 (1904)
A sheet iron vessel, A, with riveted joints and a double bottom, has a grooved rim B into which fits a cover C. The cover has three tubular openings fitted with rubber stoppers-one D is an inlet for ammonia, another E is the outlet, and the third is for a thermometer F. The ammonia inlet-tube leads into a nickel dish G which contains clean dry sodium, and stands upon a little tripod to prevent direct contact with the hot bottom of the iron vessel A. When the lid C is is position, the annular space of the rim is packed with sand. The vessel A rests on a thick iron plate heated by a suitable burner. The ammonia is dried in a tower of soda-lime; and the sodium heated to 100° or 400°. Hydrogen mixed with the excess of ammonia escapes. The reaction is represented 2NH3 + 2Na ==> 2(NH2Na)+ H2