(Chief Bee)
01-19-03 17:46
No 399715
(Rated as: excellent)
Isocoumarins: Part IIIc Synthesis of Methyl- & Methylenedioxydihydroisocoumarins & a New Synthesis of (±)-5-Methylmellein
Bhaskar H. Bhide & (Miss) Kashmira K. Shah
Indian Journal of Chemistry, 19B, 9-12 (1980)
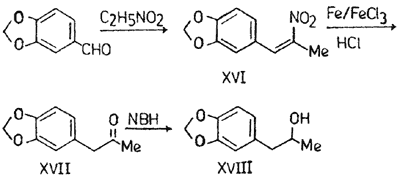
2-Nitro-1-(3,4-methylenedioxyphenyl)-1-p
Piperonal (35 g), nitroethane (50 ml), ammonium acetate (14 g) and glacial acetic acid (150 ml) were mixed and refluxed for 4 to 5 hr. The reaction mixture was then poured into ice-cold water. The fine golden yellow crystals of XVI obtained were recrystallised from rectified spirit, mp 97-98°C; 37.2 g (77%).
1-(3',4'-Methylenedioxyphenyl)-2-propano
XVI (20 g) was dissolved in hot water (200 ml) and ethanol (200 ml). Iron powder (40 g), ferric chloride (1.6 g) and conc. HCl (10N, 24 ml) were added to it very slowly with constant heating and stirring. The mixture was refluxed with continuous stirring for 6 to 8 hr and concentrated to half the volume. The precipitated black iron oxide was filtered and washed with hot water and benzene. The combined washings were extracted with benzene and dried (Na2SO4). XVII was obtained as brown oil after distilling benzene, and purified by distillation, bp 283-85°C; 10g (59%); oxime derivative, mp 86-87°C; DNP derivative, mp 138°C.
beta-Oxy-alpha-(3',4'-methylenedioxyphen
NaBH4 (5 g) was added slowly with stirring to a solution of XVII (4.3 g) in methanol (50 ml). The mixture was stirred for 16 hr. Water was added and the solution extracted with CH2Cl2. After drying (Na2SO4) it was distilled to give XVIII as dark brown oil which was purified by distillation under reduced pressure, bp 125°C/25mmHg, yellowish oil; 3.624 g (83.45%).
* J.M. Pepper, M. Saha, Can. J. Chem. 42, 13 (1964)
(Hive Bee)
01-19-03 19:31
No 399729
Finally a simple conversion of MDP2NP to MDP2P with OK yields. I was recently looking into this...Just what i was looking for!
I suspect its possible to extract with DCM instead of benzene when extracting the MDP2P? Also, it looks like they distilled the MDP2P at 260mm HG. Is this even possible? Wouldnt it polymerize? I assume that yields would increase if you distilled at a lower vacuum.
Hmm I suppose its very possible now to make MDMA from piperine! This is great if you want MDMA or MDA from the starting material, piperonal. Wow, i really need to start extracting piperine from pepper now!
Thanks Rhodium!
Sink or SWIM
(Chief Bee)
01-19-03 20:55
No 399741
I suspect its possible to extract with DCM instead of benzene when extracting the MDP2P?
It is possible to use DCM, but it is not recommended - the post-reduction mixture containing all those iron salts is very emulsion-prone as is, and would become horrible together with DCM. Better use toluene or ether, they are more hydrophobic and separates faster from the aqueous phase.
Also, it looks like they distilled the MDP2P At 260mm HG. Possible Is this even? Wouldnt it polymerize? I assumes that
yields would increase yew you distilled At has lower vacuum.
Yes, it is possible, if your ketone is reasonably pure: Post 318578 (Chromic: "dreams of MDP2P", Methods Discourse) However, good yields cannot be guaranteed - better stick to vacuum distillation.
(Hive Bee)
01-22-03 23:01
No 400521
if one were to obtain piperine, could he go through the following steps to obtain piperonal which then could be used for this synth?
piperine -> piperidine (via reflux in KOH) -> piperonal (via KMnO4 or via NaNO2 + HCl)
From reading 3base's info, I'm having trouble understanding if piperidine or piperic acid is needed for the cleavage step.
Thanks!
(Hive Addict)
01-22-03 23:23
No 400528
You need piperic acid for cleavage to piperonal. Look at the structure of piperine.
When it gets refluxed in NaOH, the amide bond at the nitrogen in the piperidine molecule gets hydrolyzed, and you are left with both piperidine and piperic acid.
But in order to get piperonal, you need to cleave the double bond right next to the phenyl ring and be left with an aldehyde. That's what's happening in the cleavage reaction, the double bond between the two carbons is being replaced with a double bond with an oxygen.
It is seductive, way too seductive. -Eleusis