02-06-03 01:07
No 405016
This could be a novel route to phenethylamines. Formation of the boronic ester from an aryl halide, running this coupling reaction and then reduce with LAH or NaBH4/H2SO4.
Abstract
A Suzuki-type cross-coupling of aryldioxaborolane with 2-bromo-N,N-dimethylacetamide in the presence of a catalytic amount of tricyclohexylphosphine as the ligand and hydroquinone as the free-radical scavenger has been demonstrated as a convenient and simple way for the synthesis of alpha-arylacetamides.
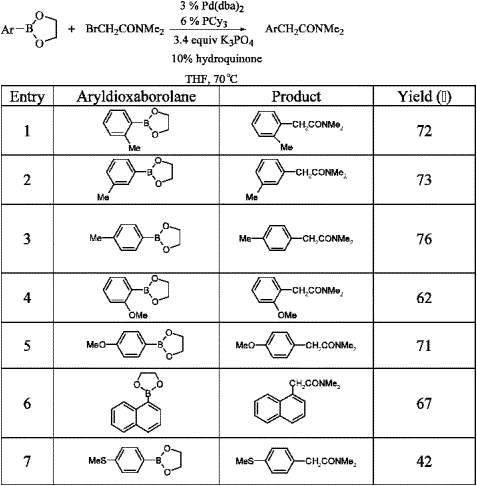
Reference: Tetrahedron Letters Volume 44, Issue 8, Pages 1587-1590 (2003) (../rhodium/pdf /pd.arylaceta