(Stranger)
07-18-03 17:10
No 448286
(Rated as: excellent)
New Chemical Cross-Coupling between Aryl Halides and Allylic Acetates Using a Cobalt Catalyst
Organic Letters, 5(7), 1043-1045 (2003)
DOI:10.1021/ol0340641
Abstract
The cobalt-catalyzed coupling reaction of aromatic halides and allylic acetates proceeds readily under mild conditions in the presence of the appropriate reducing agent to produce allylaromatic derivatives either in pure acetonitrile (aryl bromides) or in acetonitrile/pyridine mixture (aryl chlorides).
In a typical procedure to MeCN (20 mL) was successively added Zn dust (3.25 g, 50 mmol), CoBr2 (0.657 g, 3 mmol), ZnBr2 (0.338 g, 1.5 mmol), and PhBr (0.16 g, 1.5 mmol). The reaction medium was activated by adding AcOH (0.024 g, 0.4 mmol) and stirred for 30 min at rt until PhBr was totally consumed. Allyl acetate (3.03 g, 30 mmol) and ethyl 4-bromobenzoate (2.427 g, 15 mmol) were then introduced into the soln. After being stirred for 3 h at rt, the reaction mixture was poured into a soln of 2M HCl (40 mL) and extracted with Et2O (3x40 mL). The combined extracts were dried over MgSO4. Evapn of Et2O and purification by CC on SiO2 afforded 1.852 g (65%) of ethyl 4-(2-propenyl)benzoate.
AcOH activates Zn dust. Addition of ZnBr2 is not necessary but enhances the yield of the rxn. PhBr is added to decrease the amount of the reduction product (ArH). The second byproduct is Ar-Ar. The yields are within 31-75% (ArBr) and 15-83% (ArCl). Aryl zinc is postulated to be the rxn intermediate.
Supporting info: experimental details & characterization data. (http://pubs.acs.org/subscribe/journals/
For alternative methods see Post 320892 (Rhodium: "Allylbenzenes by Suzuki Coupling", Novel Discourse) ( >1 year old post, so I couldn't reply to it).
(Hive Bee)
05-01-04 18:13
No 504188
(Rated as: good read)
Cobalt-catalyzed electrochemical vinylation of aryl halides using vinylic acetates
Paulo Gomes, Corinne Gosmini and Jacques Perichon
Tetrahedron, 2003, 59, 2999-3002

DOI:10.1016/S0040-4020(03)00404-6
Abstract
The electroreduction of aryl halides (bromides or chlorides) allows the coupling reaction with vinylic acetates, in the presence of 2,2'-bipyridine and catalytic amounts of cobalt bromide, leading to styrene derivatives in good yields.
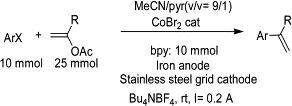
[...]
4.1. General procedure for the coupling of aryl bromides with vinylic acetates
In an undivided cell using a consumable iron anode and a stainless steel grid as the cathode, CoBr2 (0.219 g, 1 mmol), 2,2'-bipyridine (1.56 g, 10 mmol), ArBr (10 mmol) and vinylic acetate (25 mmol) were placed in a mixture solvent of acetonitrile/pyridine (45 mL/5 mL). The ionic conductivity of the medium is ensured by addition of NBu4BF4 (0.165 g, 0.5 mmol) as supporting electrolyte. The solution was electrolyzed under argon at room temperature at constant current intensity of 0.2 A (0.01 A/cm2) until the aryl bromide is totally consumed. The solution was hydrolyzed with HCl (2N) and extracted with diethyl ether, the organic layer washed with brine, dried and the solvent evapored under vacuum. Coupling products were isolated by column chromatography on silica gel with pentane/ether as eluent.
4.2. General procedure for the coupling of aryl chlorides with vinylic acetates
The procedure used for aryl chlorides is the same that aryl bromides excepted for amount of CoBr2 (0.438 g, 2 mmol).
The tendency is to push it as far as you can